Immunotherapies for cancer aim to induce the immune system to combat cancer cells more effectively. Liping Qiu, Hunan University, Changsha, China. and Hangzhou Institute of Medicine (HIM), Chinese Academy of Sciences, Zhejiang, and colleagues have developed a new, modular strategy for T-cell-based immunotherapy that manages to work without complex genetic modifications. Modulation of cell-cell communications through a regulatory circuit using various small, specially folded DNA molecules (aptamers) causes cancer cells to directly activate their mortal enemies, T cells.
Intercellular Communication
For multicell organisms like our bodies to function correctly, the cells must “coordinate” with each other. In this complex communications network, signals are sent and received, processed, and passed on. The regulation of specific membrane receptors that bind to signal molecules plays an important role in this process.
In a typical example, components of the immune system, known as antigen-presenting cells (APCs), sense the presence of cancer antigens. They transmit the signal to lymph nodes, in which specific T cells are activated by their receptors, move into the bloodstream, and kill the cancer cells. Unfortunately, cancer cells use a variety of “loopholes” to escape the immune system.
A Shortcut to T-Cell Activation
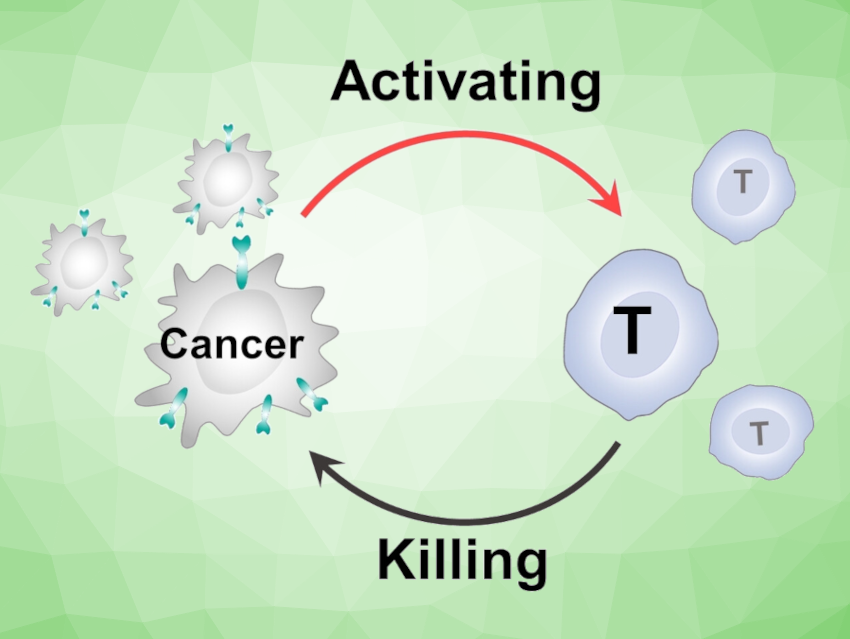
The team aimed to close these loopholes. Their goal is to establish new cellular interactions without having to produce genetically modified immune cells or receptors. The idea is to produce a “short circuit” in the communication pathways, by which the T cells are activated directly by the tumor cells, avoiding the detour through APCs.
The researchers developed a regulatory circuit consisting of two modules: “recognition-then-triggering” (module 1) and “aggregation-then-activation” (module 2). The circuit is based on different DNA aptamers, i.e., short DNA segments that fold into a “preprogrammed” 3D structure and recognize specific target molecules.
Cancer Recognition Induces Receptor Aggregation
The DNA for module 1 is initially inactive and partially paired into a double strand. If cancer cells are present, the aptamer portion of the “recognition” single strand binds to protein tyrosinase kinase 7, a protein found in large numbers on the surface of many cancer cells. This splits the DNA double strand, releasing the “triggering strand” (pictured in yellow), which triggers module 2.
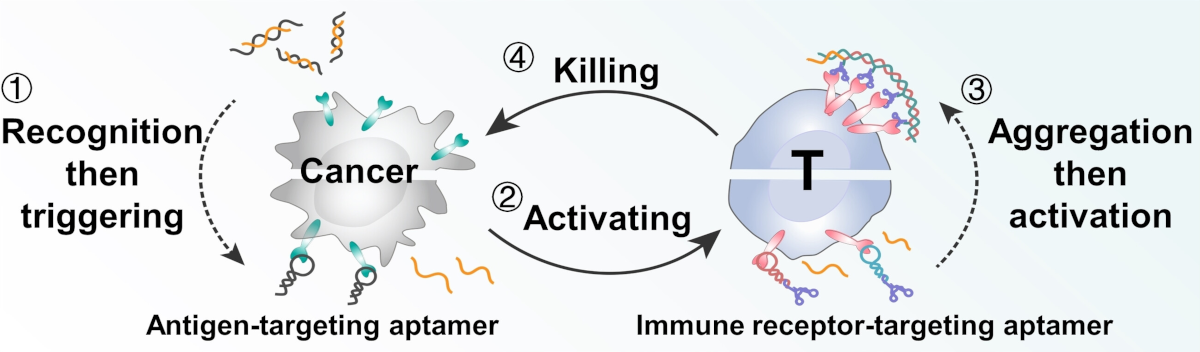
Module 2 requires two more types of aptamer. Both specifically bind to CD28 immunoreceptors on the surfaces of T cells. CD28 is a co-stimulator in the activation of T cells.
The triggering strand binds to an additional “loop” on the first aptamer. The loop opens and the newly released end binds to the second aptamer, which then binds another aptamer of the first type, and so on.
This causes the bound CD28 receptors to aggregate, triggering a signal cascade that massively amplifies the activation of T cells. In this way, “short-circuited” cell communication causes cancer cells to very effectively directly induce T cells to kill them.
- An Aptamer‐Functionalized DNA Circuit to Establish an Artificial Interaction between T Cells and Cancer Cells,
Zhimin Wang, Yue Zhang, Limei Wu, Jianghuai Chen, Sitao Xie, Jiaxuan He, Qiang Zhang, Hong Chen, Fengming Chen, Yue Liu, Yutong Zhang, Yuting Zhuo, Nachuan Wen, Liping Qiu, Weihong Tan,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202307656