Cyclic (alkyl)(amino)carbenes (CAACs) are useful, e.g., as ligands in homogenous catalysis. They outperform the well-known N-heterocyclic carbenes (NHCs), which can be considered cyclic diaminocarbenes, in several catalytic reactions. The steric and electronic properties of CAACs can be tuned by changing the substituents.
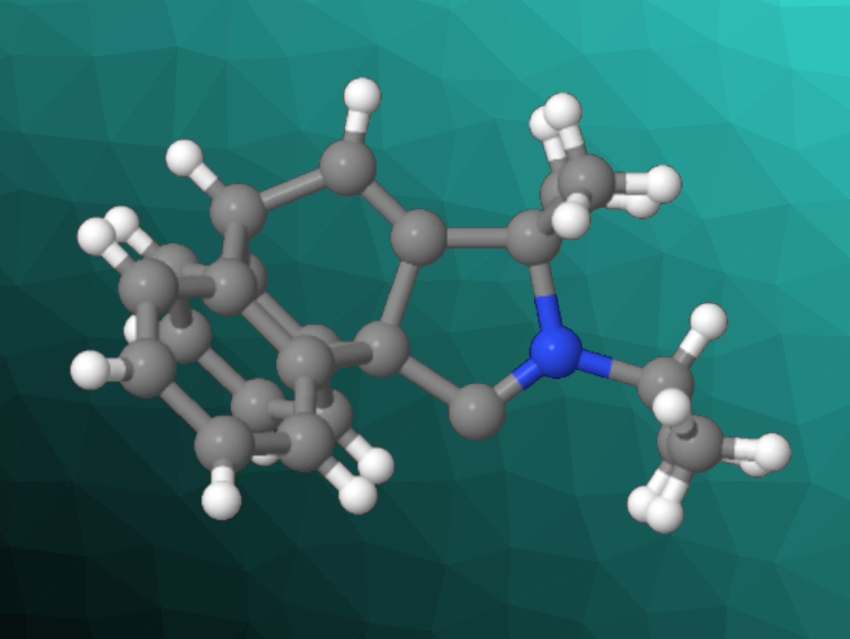
Guy Bertrand, University of California, San Diego, La Jolla, USA, and colleagues have synthesized a new family of CAACs, which the team called cyclic (amino)(barrelene) carbenes (CABCs, general structure pictured below). The key step in the preparation of these carbenes is an intramolecular [4+2] cyclization of an anthracene derivative with an amine-functionalized alkyne.
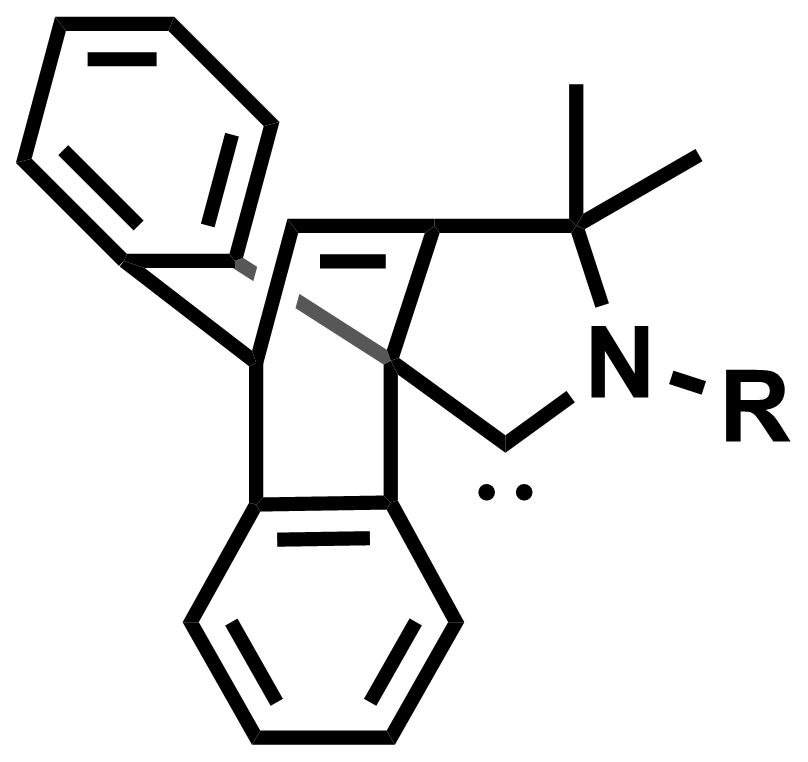
A representative synthesis of CABCs starts with the condensation of 9-anthraldehyde with 1,1-dimethyl-propargyl amine, followed by the [4+2] cycloaddition and alkylation or arylation at the nitrogen atom. The resulting iminium salts can be deprotonated, e.g., with potassium bis(trimethylsilyl)amide (KHMDS) to obtain the free carbenes.
The barrelene-based structure flanking the carbene has two hydrogen atoms that could interact with a metal center in CABC complexes. The team prepared CABC copper and gold complexes and confirmed interactions between barrelene hydrogens and the metal centers using 1H NMR spectroscopy. According to the researchers, this interaction could stabilize low-valent catalytic intermediates.
- Cyclic (amino)(barrelene)carbenes: an original family of CAACs through a novel synthetic pathway,
Melinda R. Serrato, Mohand Melaimi, Guy Bertrand,
Chem. Commun. 2022.
https://doi.org/10.1039/d2cc02565e