Molecules that selectively bind anions are essential in diverse areas of chemistry, for example, for analyzing anion levels in biological specimens, catalyzing reactions, and beverage testing. Sulfate anions (SO42–), in particular, are implicated in all of these areas, and being able to remove them selectively would also be beneficial in nuclear waste treatment. However, there are significant challenges involved in binding sulfate anions in solvents with a high water content. This is due to the solvation sphere which forms around each sulfate anion and is energetically very difficult to disrupt.
Evelyne Deplazes, The University of Queensland, St. Lucia, Australia, Xin Wu, Xiamen University, China, and The University of Queensland, and colleagues have developed a urea-based organic cage for binding sulfate anions that is effective in much higher water concentrations than have previously been attainable. Not only is the team’s cage (a cryptand) better at binding sulfate anions than most others currently available, it can also be prepared in a one-pot process. Previous comparably efficient cages have taken up to 20 steps to produce.
Hydrogen Bonding for Sulfate Capture
The team took a bio-inspired approach to finding better, more efficient molecular cages. Sulfate-binding proteins are found throughout nature and serve essential roles in anion transport and isolation. Unlike synthetic systems, they do this in the absence of positive charges nearby to stabilize the anions, relying solely on hydrogen bonding.
This is hard to replicate in synthetic aqueous environments, which usually need polycationic hosts to provide strong attraction to the anion of interest, allowing it to be bound selectively in environments containing a number of different anions.
One-Step Templated Synthesis
Previous studies have produced molecules that are able to selectively bind anions under these conditions. However, they are complex to produce, not sufficiently selective, and generally ineffective at water concentrations of over 70 %. For example, cyclopeptides that wrap around sulfate anions require at least ten steps to produce, some other cages formed using dynamic covalent bonds are not stable, and yet others are not produced in viable yields.
The strategy of producing a molecular cage can improve binding strength and selectivity, and so the team harnessed this approach. They produced their cage using a one-pot process, heating a mixture including a carbonyl-imidazole compound, ethylenediamine, and tetrabutylammonium sulfate as a template to help form cages with the “correct” shape. The sulfate was then precipitated out of the mixture using barium chloride, leaving an empty cage ready for sulfate binding.
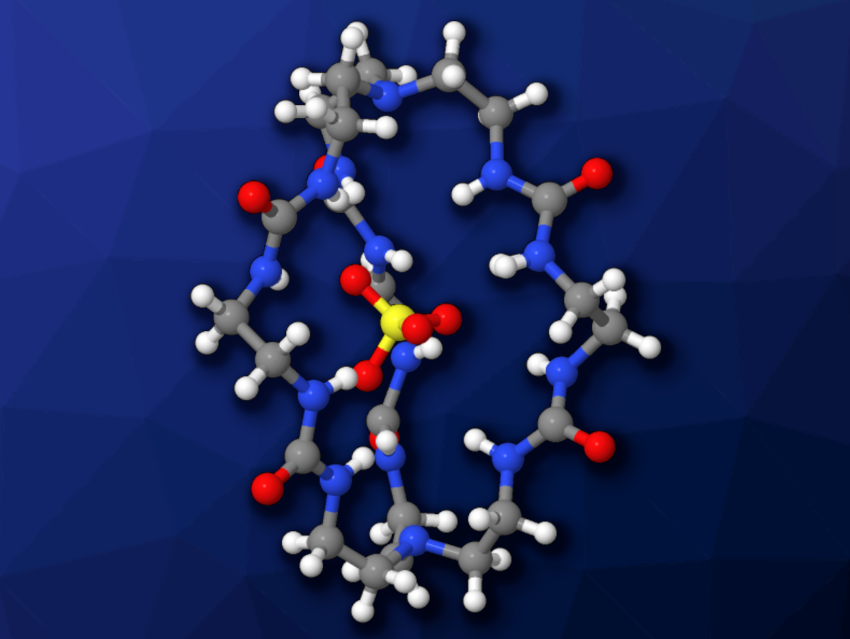
The team’s cage is composed mainly of urea, originating from the carbonyl-imidazole compound. The six urea units within the cage can form 12 strong hydrogen bonds with an encapsulated sulfate anion (“filled” cage pictured).
Sulfate Binding Affinity Enhanced by Micelles
The team tested their cage at high water concentrations and found that the sulfate binding affinity remained high and the binding was energetically favorable, even taking into account the extra energy required for desolvation under these conditions. The previously most potent water-soluble anion receptor has a high sulfate affinity, but requires 20 steps to produce.
The team was inspired by this receptor’s efficiency due to the hydrophobic microenvironment in which anion binding occurs. The team found that the binding affinity was dramatically enhanced in the presence of CTAB (cetyltrimethylammonium bromide) micelles, even though the cages do not enter the micelles until they have bound a sulfate anion. They explain that this is likely due to the electrostatic interactions between different parts of the CTAB molecule and the charges on the sulfate-bound cage.
Potential Uses of the New Molecular Cages
The researchers have proposed uses for their sulfate-binding cage in analyzing water samples and beverage samples. They have shown that successful sulfate ion analysis takes 2–3 minutes using their cage in these cases, as opposed to 20–30 min for ion chromatography analysis.
Thus, the team achieved time savings not only in cage synthesis but also in its use, providing an efficient and inexpensive way to analyze and isolate sulfate ions, and allowing the use of the cages at much higher water concentrations. This could expand the range of potential uses in industry, biology, and the environment.
- A charge-neutral organic cage selectively binds strongly hydrated sulfate anions in water,
Liuyang Jing, Evelyne Deplazes, Jack K. Clegg, Xin Wu,
Nat. Chem. 2024.
https://doi.org/10.1038/s41557-024-01457-5