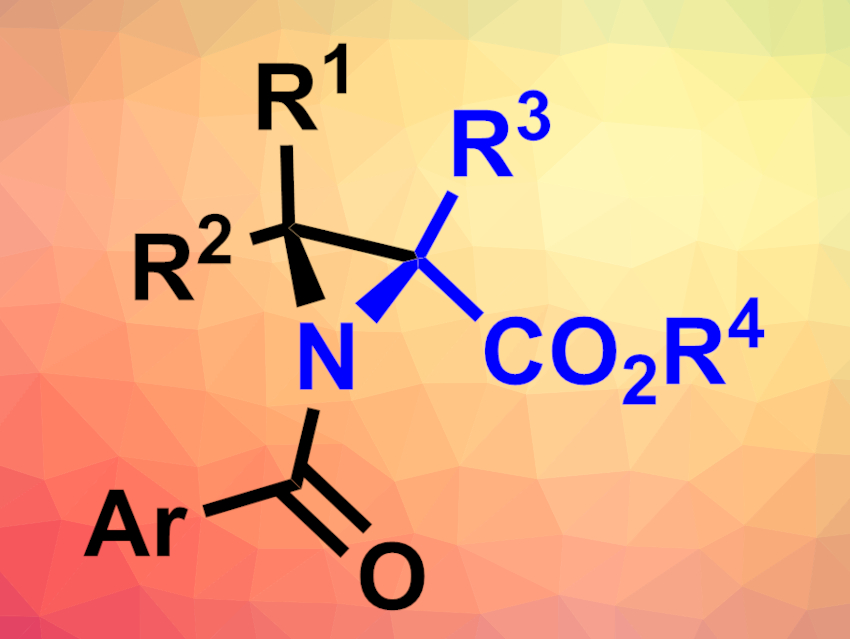
Synthesizing organic compounds with small, strained, highly substituted rings can be challenging. Preparing a larger ring first, which is then contracted, could be a workaround for this problem. The quasi-Favorskii rearrangement is one such ring-contraction reaction. However, there had been no variant of this reaction for nitrogen-containing rings so far, e.g., for the preparation of aziridines, which are three-membered heterocycles with one nitrogen atom.
Juha H. Siitonen, Rice University, Houston, TX, USA, and Aalto University, Espoo, Finland, Daniel H. Ess, Brigham Young University, Provo, UT, USA, László Kürti, Rice University, and colleagues have developed a nitrogen analogue of the quasi-Favorskii rearrangement, the aza-quasi-Favorskii rearrangement (overall reaction pictured below). Using this reaction allowed them to synthesize highly substituted aziridines (general structure pictured above).
The team started from a ketone as an enolate precursor, which reacts with a ketomalonate-derived N-electrophilic reagent in a Mannich reaction to give an β-aminoketone-type compound. This precursor then cyclizes to the corresponding anionic azetidine (a four-membered ring). The four-membered ring undergoes the desired aza-quasi-Favorskii rearrangement and gives substituted aziridines.
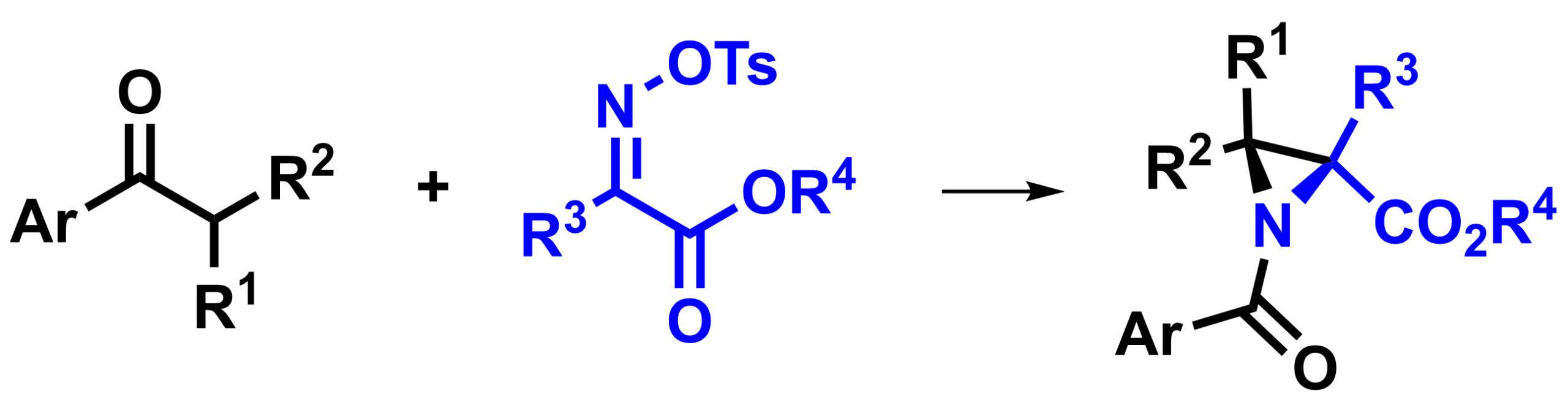
The desired aziridine products were obtained in moderate to good yields in many cases. The reaction was also used to prepare strained (hetero)spirocyclic systems. The aziridines can be useful in further transformations.
- Aza-Quasi-Favorskii Reaction: Construction of Highly Substituted Aziridines through a Concerted Multibond Rearrangement Process,
Padmanabha V. Kattamuri, Jidong Zhao, Tamal Kanti Das, Juha H. Siitonen, Nathan Morgan, Daniel H. Ess, László Kürti,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c03805