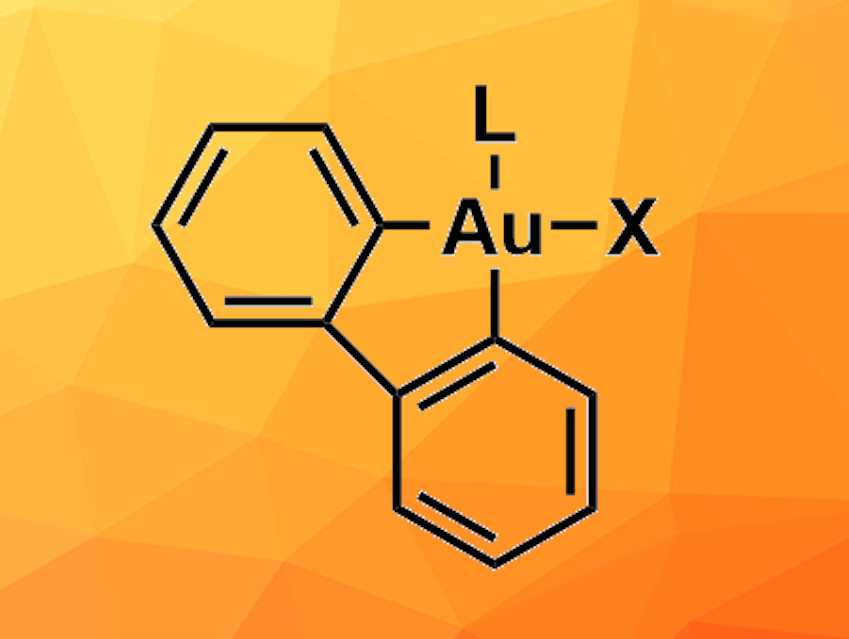
2,2′-Biphenyl gold(III) complexes can be useful, e.g., in catalysis or for investigations of the chemistry of gold(III) compounds. Established synthesis methods for these compounds generally use dibenzostannoles that transfer the biphenyl ligand to the gold precursor via a transmetallation process. However, organotin compounds can be highly toxic and difficult to store because they degrade under light and oxygen. Therefore, alternative approaches for the synthesis of 2,2′-biphenyl gold(III) complexes are desirable.
A. Stephen K. Hashmi, Heidelberg University, Germany, and colleagues have developed a synthesis route for 2,2’-biphenyl gold(III) complexes using dibenzosilol as a biphenyl precursor. Na[AuCl4] was used as the gold precursor and mixed with dibenzosilol and silver fluoride in acetonitrile overnight at 80 °C. An oligomeric intermediate was formed, which was then reacted with different ligands in tetrahydrofuran (THF) at room temperature to obtain the target complexes. This protocol was tested using six different ligands and provided the target products in moderate to good yields—except for a very bulky phosphite ligand, for which no product was observed.
One of the product complexes, which contains dimethyl sulfide as an additional ligand, was used as a precursor for the synthesis of 2,2′-biphenyl gold(III) complexes with carbene ligands. This reaction could be used to introduce different carbenes, and the yields varied between 38 % and 92 %, depending on the steric bulk of the carbene ligand. The dimethyl-sulfide-containing complex was also used in the synthesis of a gold(III) carbene complex via intermediate formation of an isonitrile complex—a route that can provide access to otherwise difficult-to-obtain products. Overall, the developed synthesis route can be used for the synthesis of a variety of different 2,2′-biphenyl gold(III) complexes and could be a useful alternative to the tin-based approach.
- Tin-Free Synthesis of 2,2′-Biphenyl Gold(III) Complexes Using Dibenzosilol,
Alexander Ahrens, Leonhard F. P. Karger, Frank Rominger, Matthias Rudolph, A. Stephen K. Hashmi,
Organometallics 2023.
https://doi.org/10.1021/acs.organomet.3c00144