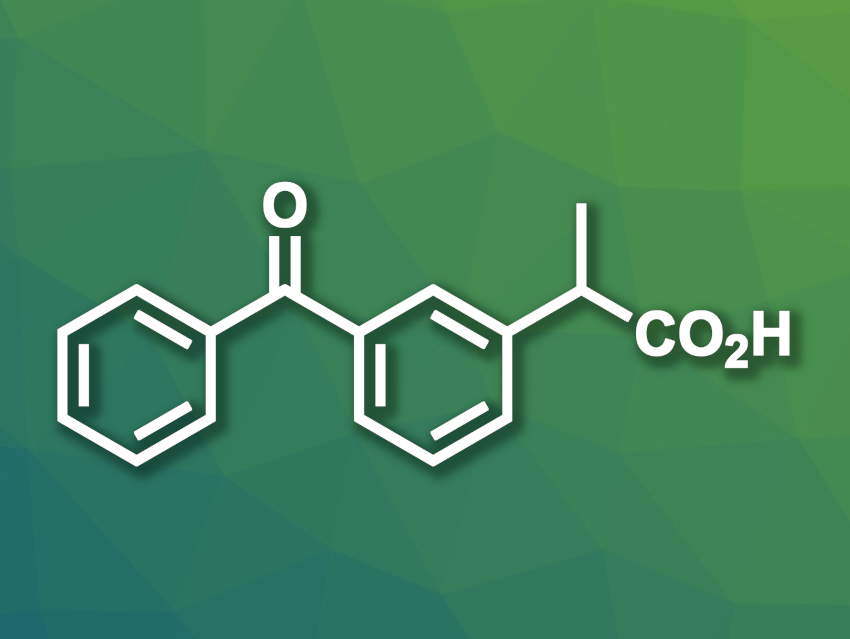
Ketoprofen is a nonsteroidal anti-inflammatory drug (NSAID) with a 2-arylpropionic acid unit. It is efficient in low doses and is approved for both human patients and for use in veterinary medicine. Different industrial processes for the synthesis of ketoprofen are available, but they generally depend on hazardous or harmful reactants such as sodium amide or benzene. Greener and more efficient routes for the preparation of ketoprofen would be useful.
Pei Tang, Sichuan University, Chengdu, China, Fener Chen, Fudan University, Shanghai, China, Shanghai Engineering Research Center of Industrial Asymmetric Catalysis of Chiral Drugs, and Sichuan University, and colleagues have developed a five-step synthesis route for ketoprofen with an overall yield of 44 %. The synthesis starts from cyclohexanone, which undergoes a Stork enamine alkylation with pyrrolidine. The enamine is then reacted with benzyl chloride and hydrolyzed using dilute hydrochloric acid to give benzyl cyclohexanone.
The benzyl cyclohexanone is then converted to a dihydrobenzofuranone intermediate via an aldol addition/intramolecular enol-lactonization cascade, using titanium tetrachloride in the presence of triethylamine in dichloromethane. The resulting intermediate undergoes pyrolytic aromatization at 230–250 °C in the presence of pyridinium hydrochloride to give an arylpropionic acid derivative. Finally, a benzylic oxidation gave the desired ketoprofen.
The team performed the synthesis at the decagram scale with good yields. The approach does not require toxic solvents and it could be a useful alternative for the industrial synthesis of ketoprofen.
- Efficient and Scalable Synthesis of Ketoprofen: A Pyrolytic Aromatization Approach,
Lili Song, Zhigang Liu, Minjie Liu, Pei Tang, Fener Chen,
Org. Process Res. Dev. 2023.
https://doi.org/10.1021/acs.oprd.3c00049