Hydrogen could be useful as an environmentally friendly fuel. Metal hydrides, such as nickel hydride complexes, are interesting for hydrogen production due to their potential for the transfer of protons, hydrogen atoms, or hydride ions.
Karsten Meyer, Universitäty of Erlangen-Nürnberg, Erlangen, Germany, and colleagues have prepared a zero-valent nickel complex with a tris-N-heterocyclic carbene ligand that can undergo a two-step protonation reaction leading to H2 evolution. The complex, i.e., [Ni(TIMENi-Pr)], was prepared via a reaction of [Ni(COD)2] (COD = 1,5-cyclooctadiene) with the ligand TIMENi-Pr in tetrahydrofuran (THF).
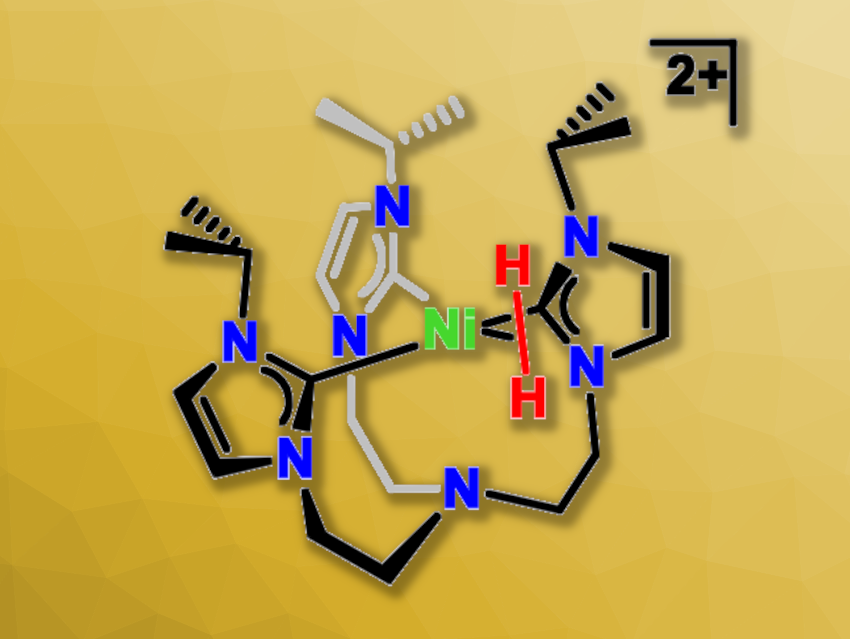
The team then protonated the complex using a lutidinium salt, i.e., [2,6-lutidinium](BPh4). They obtained the Ni(II) monohydride complex [(TIMENi-Pr)Ni(H)]+. Adding another equivalent of the lutidinium salt to this product led to the evolution of H2 bubbles and gave the Ni(II) species [(TIMENi-Pr)Ni(NCCH3)]2+, which was isolated from an acetonitrile solution. This second protonation involves a rare nickel dihydrogen complex (pictured) as an intermediate. The Ni(II) acetonitrile complex can be reduced to regenerate the Ni(0) precursor, allowing for an H2-evolving closed cycle.
- Closed Synthetic Cycle for Nickel‐Based Dihydrogen Formation,
Soosan Hosseinmardi, Andreas Scheurer, Frank W. Heinemann, Nicola Marigo, Dominik Munz, Karsten Meyer,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202302063