The enantio- and diastereoselective introduction of two nonadjacent stereocenters in 1,3-positioning at the same time using a single catalyst can be a challenge in organic synthesis, especially if the two stereocenters are not located in the same ring within a cyclic product. Nickel-catalyzed reductive cross-coupling reactions using alkenes and primary alkyl halides can be performed in an enantioselective manner, but they generate only a single stereocenter. So far, there had been no way to use them to selectively create 1,3-type nonadjacent stereocenters using secondary alkyl electrophiles.
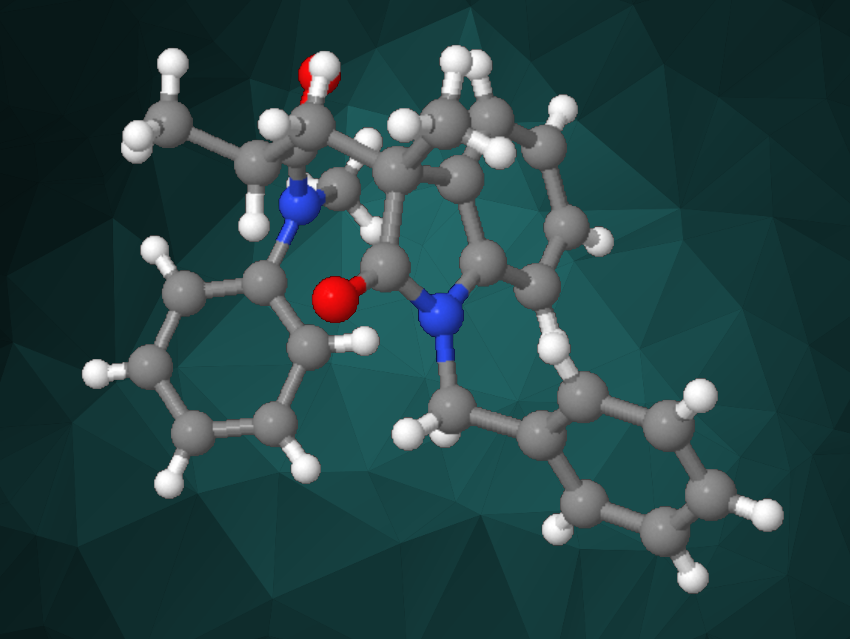
Minyan Wang, Nanjing University, China, Junliang Zhang, Fudan University, Shanghai, China, Wangqing Kong, Wuhan University, China, and colleagues have developed an approach to the Ni-catalyzed reductive cyclization/cross-coupling of alkene-tethered aryl bromides and α-bromoamides, giving oxindoles containing nonadjacent stereocenters (example product pictured) in a stereoselective manner.
The team used NiBr2(DME) as the catalyst (DME = 1,2-dimethoxyethane) together with a chiral sulfinamide monophosphine (Ming-Phos) ligand for the reaction of various alkene-functionalized aryl bromides with different α-bromo amides, which act as secondary alkyl precursors. The reactions were performed using Zn as a reducing agent, MgCl2 and LiI as additives, and tetrahydrofuran (THF) as the solvent.
The researchers obtained the desired oxindoles in moderate to good yields and with high levels of enantioselectivity and diastereoselectivity. In addition, they found that the same starting materials can be used to prepare a set of complementary products (diastereomers of those obtained using the Ming-Phos ligand). For this, the team employed a phosphinooxazoline ligand (Ph-Phox).
- Ligand-Controlled, Nickel-Catalyzed Stereodivergent Construction of 1,3-Nonadjacent Stereocenters,
Qi Pan, Kuai Wang, Weipeng Xu, Yuqi Ai, Yuanyuan Ping, Chuhan Liu, Minyan Wang, Junliang Zhang, Wangqing Kong,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c03745