Nickel(I) complexes are interesting research targets with applications in catalysis and importance in biological systems. Due to the odd electron number at the nickel center, Ni(I) complexes often form dimeric structures with a Ni−Ni bond. Mononuclear and cationic Ni(I) complexes are, rare, especially with unsupported neutral arene ligands.
Ingo Krossing, University of Freiburg, Germany, and colleagues have synthesized Ni(I) half-sandwich and sandwich cations (examples pictured). The team prepared half-sandwich cations of the type [Ni(arene)(CO)2]+ (arene = C6H6, C6H4F2) and the sandwich cation [Ni(C6H4F2)2]+ from [Ni(CO)4][F{Al(ORF)3}2] (RF = C(CF3)3) via irreversible removal of CO from the equilibrium (pictured below, WCA = weakly coordinating anion).
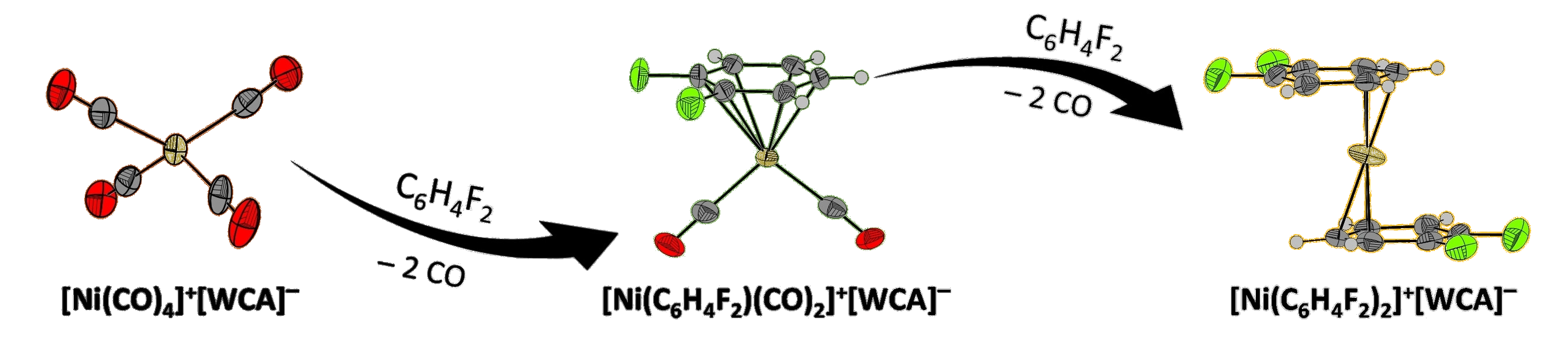
For the synthesis of the half-sandwich complexes, the team added one equivalent of the corresponding arene to a solution of [Ni(CO)4][F{Al(ORF)3}2] in 1,2,3,4-tetrafluorobenzene. A dynamic vacuum had to be applied to remove CO from the equilibrium and complete the reaction with ortho-difluorobenzene. The synthesis of the sandwich cation with two ortho-difluorobenzene units was achieved when the researchers dissolved [Ni(CO)4][F{Al(ORF)3}2] in pure ortho-difluorobenzene and the mixture was stirred for 3 d in an open reaction vessel inside a glovebox.
Overall, the work demonstrates the advantages of using [Ni(CO)4]+ as a Ni(I) synthon: Even complexes with very weak ligands such as ortho-difluorobenzene become directly accessible. According to the researchers, the procedure is likely transferable to other weak ligands.
- [Ni(CO)4]+ as NiI Synthon: Synthesis and Characterization of NiI Half‐Sandwich and Sandwich Cations,
Manuel Schmitt, Maximilian Mayländer, Valentin Radtke, Tim Heizmann, Sabine Richert, Ingo Krossing,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202301419
![[Ni(CO)4]+ Used as a Ni(I) Synthon](https://www.chemistryviews.org/wp-content/uploads/2023/06/nickelcarbonyl.png)



