The development of new energetic materials (explosives) can, e.g., lessen the environmental impact of their use. For example, they should ideally be free of heavy metals and perchlorates. Nitrogen-rich heterocycles such as tetrazoles, for example, can be useful in this context. They can be combined with groups such as azides or nitrimines to tune their properties.
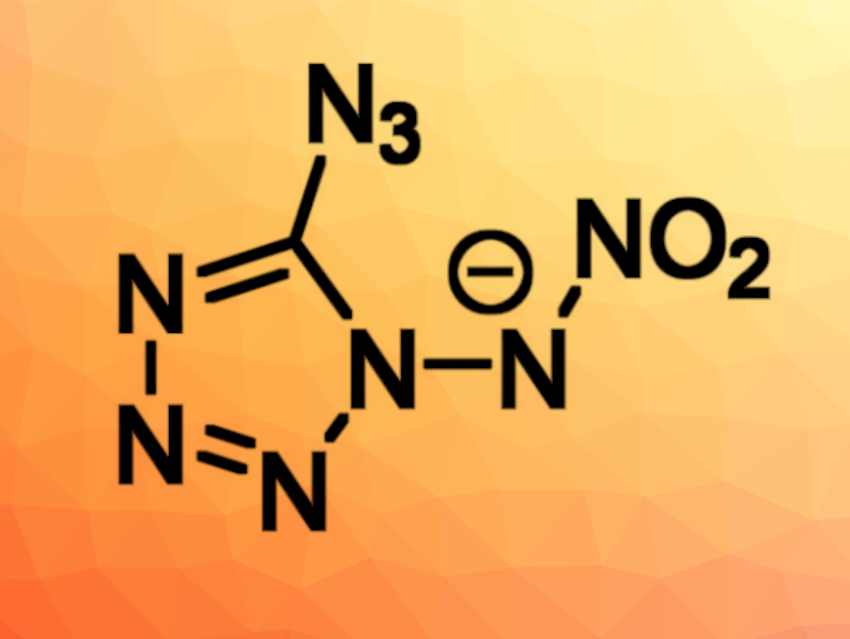
Thomas M. Klapötke, Jörg Stierstorfer, Ludwig Maximilian University of Munich (LMU), Germany, and colleagues have combined a nitrimine and an azide function in a monotetrazole derivative. They prepared 1-nitrimino-5-azidotetrazole (2, pictured below) as well as ionic derivatives of the compound (anion pictured above). The team started from triaminoguanidinium chloride, which was converted to 1-amino-5-azidotetrazole (1) using HNO3 and NaNO2. The resulting 1-amino-5-azidotetrazole was nitrated with a sulfuric acid/nitric acid (1:1) mixture at −15 °C.
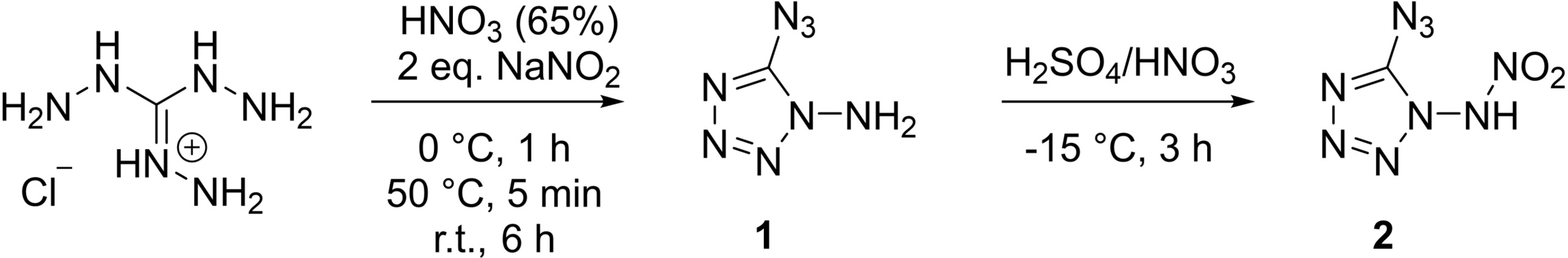
The researchers obtained 1-nitrimino-5-azidotetrazole in a yield of 54 %. They synthesized ionic derivatives of the compound, e.g., an ammonium and a guanidinium derivative via reactions with ammonia and guanidinium carbonate, respectively. According to the team, the ammonium salt shows promising properties with a combined nitrogen and oxygen content of 91.5 % and could be considered as a possible primary explosive. However, 1-nitrimino-5-azidotetrazole is one of the most energetic monoheterocycles and its sensitivity is difficult to “tame”.
- 1‐Nitrimino‐5‐azidotetrazole: Extending Energetic Tetrazole Chemistry,
Maximilian Benz, Thomas M. Klapötke, Jörg Stierstorfer,
ChemPlusChem 2022.
https://doi.org/10.1002/cplu.202200186