The oxidative addition of C–C bonds to transition metals can be an important step in catalytic processes in organic chemistry. Using a strained C–C bond, e.g., in an activated cyclopropane, can make this step easier. Nonactivated cyclopropanes are generally easier to synthesize than activated examples, but are more challenging substrates in C–C bond activation reactions.
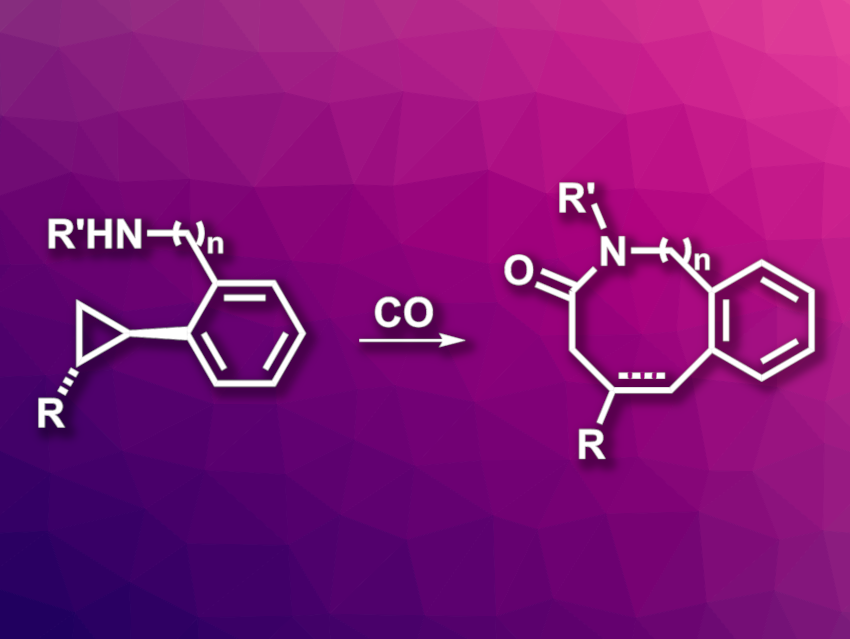
John F. Bower, University of Liverpool, UK, and colleagues have developed a regioselective C–C bond activation of nonactivated cyclopropanes (general example pictured). The team used rhodium catalysis together with secondary amines or anilines as directing groups to form amino-stabilized rhodabicycles. A carbonylative C–N bond formation then gives seven- or eight-membered lactams as products.
A variety of substituted cyclopropanes with the requisite directing groups were reacted with CO (1 atm) using [Rh(cod)2]BARF as a catalyst (cod = 1,5-cyclooctadiene, BARF = tetrakis[3,5-bis(trifluoromethyl)phenyl]borate), dimethyl fumarate as an additive/oxidant, and benzonitrile (PhCN) as the solvent. The reactions were performed at 120 °C. The desired products were obtained in moderate to high yields.
- Carbonylative N-Heterocyclization via Nitrogen-Directed C–C Bond Activation of Nonactivated Cyclopropanes,
Adam D. J. Calow, David Dailler, John F. Bower,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c02921