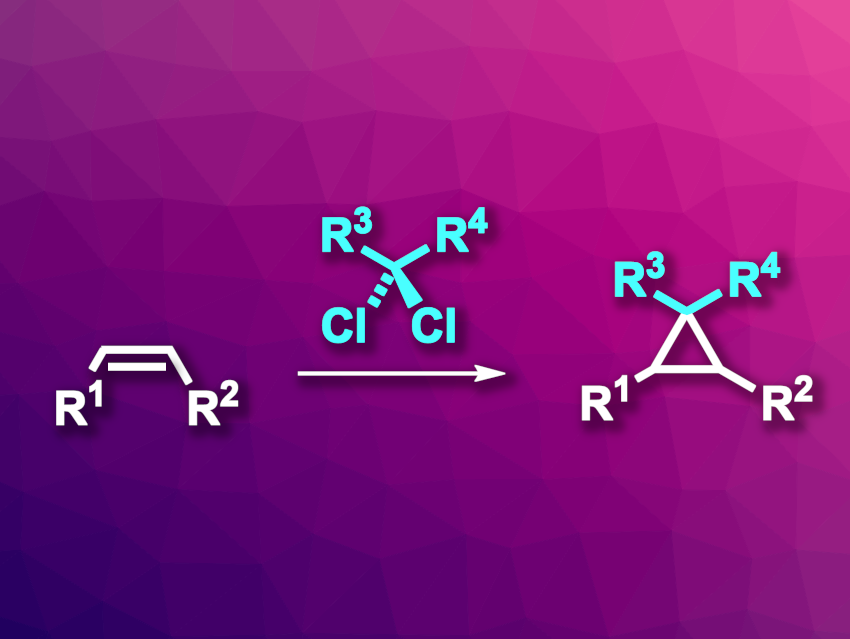
Cyclopropanes are often found, e.g., in biologically active compounds. Chiral cyclopropanes can be synthesized via a catalytic asymmetric addition of a diazoalkane to an alkene, for example. However, diazoalkanes, which serve as carbene precursors in this type of reaction, can be very reactive and can require stabilizing groups for their safe use. One alternative is the Simmons–Smith reaction, which can be used to introduce nonstabilized carbenes. However, existing catalytic asymmetric Simmons–Smith reactions often need stoichiometric amounts of a chiral Lewis acid or directing groups.
Christopher Uyeda, Purdue University, West Lafayette, IN, USA, and colleagues have developed a cobalt-catalyzed cyclopropanation approach in which gem-dichloroalkanes serve as precursors for nonstabilized carbenes (pictured). This approach avoids the limitations in substrate scope caused by the use of chiral Lewis acids. The team used an (OIP)CoBr2 complex (OIP = oxazoline iminopyridine) as a chiral catalyst together with Zn as a reductant and ZnBr2 as an additive. The reactions were performed at room temperature in tetrahydrofuran (THF).
Under these conditions, the team reacted different 1,3-dienes and monoalkenes with gem-dichloroalkanes such as 2,2-dichloropropane or gem-dichloro reagents derived from cyclic ketones. The team obtained high yields for both electron-rich and electron-deficient alkenes. Bulkier substrates led to lower yields. Substrates with a branched carbon on one side of the double bond gave the desired cyclopropanated products with generally high enantioselectivities.
- Catalytic Asymmetric Cyclopropanations with Nonstabilized Carbenes,
Kristen E. Berger, Raymond J. Martinez, Jianhan Zhou, Christopher Uyeda,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c01949