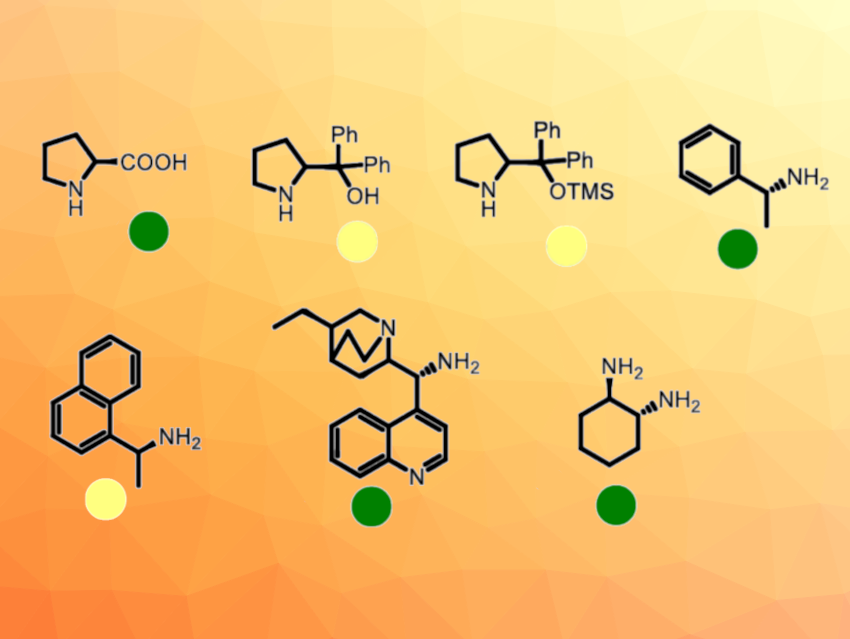
Asymmetric organocatalysis is a useful method in organic synthesis. However, there is little data on the environmental safety of organocatalysts, although this information is crucial for the sustainability of organocatalytic approaches.
Anne Kahru, National Institute of Chemical Physics and Biophysics and Estonian Academy of Sciences, both Tallinn, Estonia, Tõnis Kanger, Tallinn University of Technology, Estonia, and colleagues have used the naturally bioluminescent bacteria Vibrio fischeri as a toxicity screening tool for a library of 26 organocatalysts. These bacteria respond to toxic compounds by decreasing their bioluminescence within seconds to minutes. The light loss is proportional to the toxicity of the chemical.
The team prepared bacterial suspensions for the toxicity measurements from freeze-dried bacteria, dissolved the respective organocatalysts in dimethyl sulfoxide (DMSO), and added them to the bacterial suspensions in different concentrations. Then the luminescence was recorded over a span of 30 min.
The obtained data showed that aminocatalysts (examples pictured) have low ecotoxicity. Hydrogen-bonding catalysts, i.e., thioureas and squaramides, were found to be about ten times more toxic. More specifically, none of the studied thioureas and squaramides was ranked as “not harmful”. The presence of a trifluoromethyl moiety increased their toxic effect. Thus, thioureas and squaramides may be harmful to the environment and need a more thorough (eco)toxicological evaluation. Aminocatalysts could be considered more environmentally safe or green alternatives.
- Aminocatalysts are more environmentally friendly than hydrogen‐bonding catalysts,
Mariliis Sihtmäe, Estelle Silm, Kadri Kriis, Anne Kahru, Tõnis Kanger,
ChemSusChem 2022.
https://doi.org/10.1002/cssc.202201045
Update (July 1, 2002)
An earlier version of the article was missing part of the research team. This has been corrected.