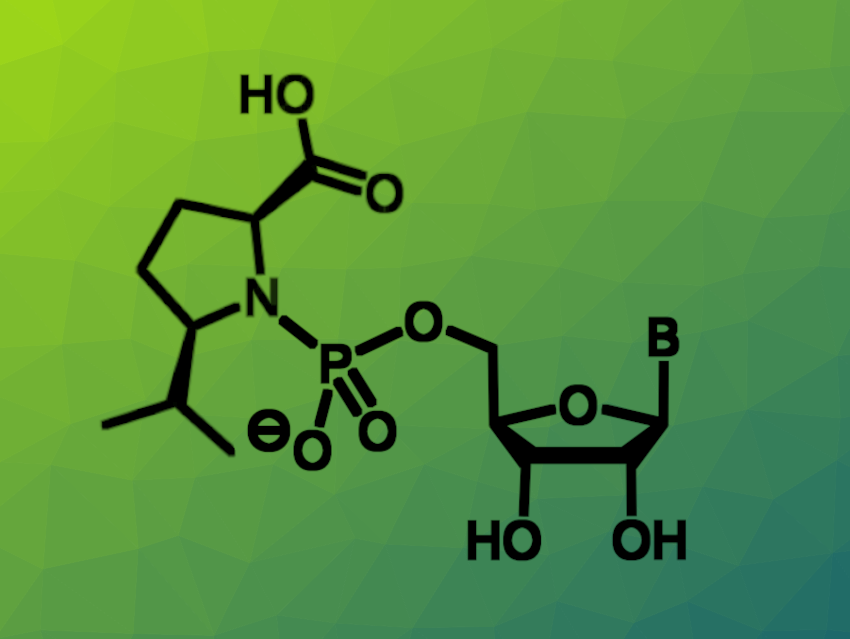
Nucleoside monophosphates (NMPs) are the building blocks of nucleic acids. Phosphoramidites of nucleosides can be converted to highly reactive monomers when an acidic organocatalyst is added during solid-phase synthesis of DNA or RNA, making them useful starting materials for oligonucleotide synthesis. In addition, nucleotide prodrugs in which the phosphate is masked as an aryl amino acidyl ester phosphoramidate can be used as antivirals. The “unmasking” of the nucleotide by hydrolysis is important in this context, and different leaving groups could be employed. Proline can act as a leaving group when it is linked to NMPs via its nitrogen atom, but the resulting level of reactivity is generally not high enough for practical purposes.
Clemens Richert, University of Stuttgart, Germany, and colleagues have investigated the effects of substitution at the proline unit on the leaving group qualities and the resulting nucleotide monomer reactivity. The team aimed to identify compounds that are more reactive than the unmodified prolinyl nucleotides. They prepared a range of substituted prolinyl nucleotides (example pictured) and related compounds and tested their reactivity in hydrolysis assays.
The researchers found prolinyl nucleotides with increased reactivity can be created by introducing additional substituents, with both alkyl and carboxyl groups leading to a higher rate of nucleotide release. For example, a δ-carboxy prolinyl NMP was converted with a half-life of just 11 min in a magnesium-free buffer, and a δ-isopropyl NMP reacted seven times faster than its prolinyl counterpart in enzyme-free genetic copying of RNA. According to the team, the proline scaffold is promising for developing leaving groups with further improved reactivity.
- Prolinyl Phosphoramidates of Nucleotides with Increased Reactivity,
Adrian Humboldt, Fabian Rami, Franka M. Topp, Dejana Arnold, Daniela Göhringer, Pradeep S. Pallan, Martin Egli, Clemens Richert,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202319958