Diaryliodonium salts are useful, environmentally friendly arylating reagents for the synthesis of various heterocycles. o-Iodoaniline and its derivatives have been used to prepare heterocycles, but diaryliodonium salts derived from o-iodoanilines have not been well-explored for their synthetic utility. Indolo-[2,3-b]indole derivatives represent a potential type of heterocyclic product. They are used, e.g., in organic light-emitting diodes (OLEDs).
Manish Kumar Mehra, Chung Whan Lee, Gachon University, Seongnam, South Korea, and colleagues have developed a method for the one-pot synthesis of various indolo[2,3-b]indoles via a copper-catalyzed transformation of 2-(substituted-amino)aryl)(mesityl)iodonium salts and indole derivatives (pictured below, PG = protecting group). The diaryliodonium salts are easily obtained from o-iodoanilines.
The reaction involves a copper-catalyzed arylation followed by an intramolecular cyclization. The team used Cu(OTf)2 as a catalyst in the presence of 2,6-di-tert-butylpyridine (d(t-bu)py) in dichloromethane (DCM) as the solvent for the arylation, followed by the addition of iodine, Cs2CO3, and acetonitrile (MeCN) for the cyclization.
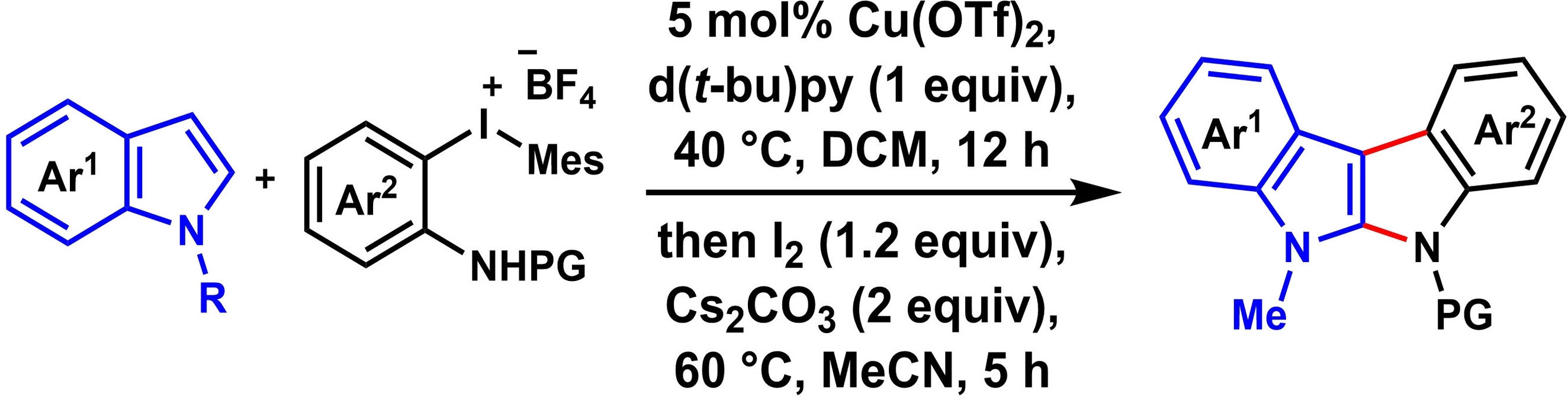
Under these conditions, a variety of diaryliodonium salts and indoles were transformed into a series of indolo[2,3-b]indole derivatives in good yields. The reaction shows a good functional group tolerance and a broad substrate scope.
- Copper‐Catalyzed One‐Pot Arylation and Cyclization of Diaryliodonium Salts Derived from o‐Iodoanilines for Indolo[2,3‐b]indoles Syntheses,
Miseon Choi, Manish Kumar Mehra, Chung Whan Lee,
Eur. J. Org. Chem. 2023.
https://doi.org/10.1002/ejoc.202201286
![One-Pot Synthesis of Indolo[2,3-b]indoles](https://www.chemistryviews.org/wp-content/uploads/2023/01/indoloindoles.png)



