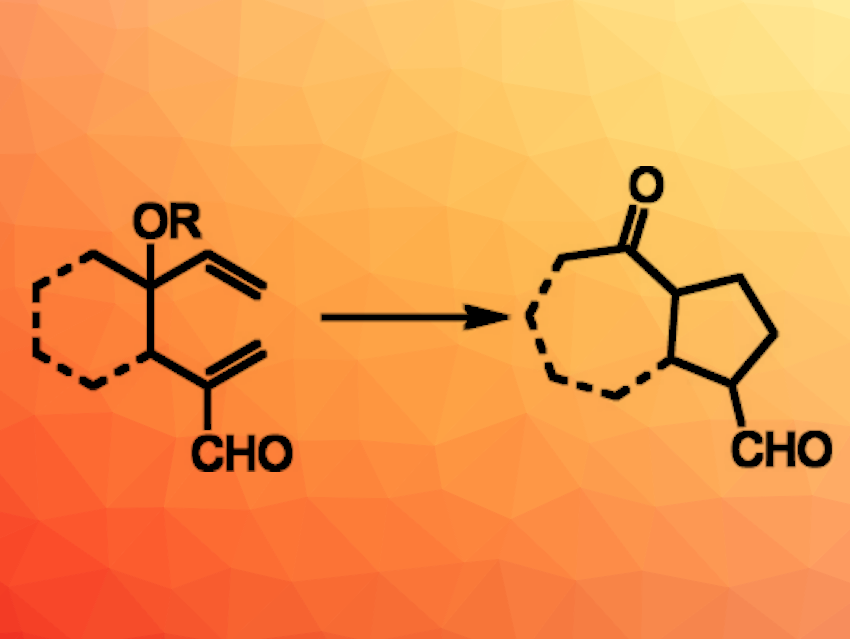
The oxy-Cope rearrangement is a [3,3] sigmatropic rearrangement of a hexa-1,5-dien-3-ol unit, a variation of the Cope rearrangement of 1,5-dienes. Cope rearrangements have been catalyzed using transition metal salts and more recently with organocatalysts. The products of oxy-Cope reactions can be used in tandem reactions, e.g., with Michael reactions.
James L. Gleason, McGill University, Montreal, Canada, and colleagues have developed an organocatalytic oxy-Cope/Michael cascade reaction (general reaction pictured). The team used ethyldiazepane carboxylate to catalyze an oxy-Cope rearrangement of 4-hydroxy- (R = H) or 4-alkoxy-1,5-hexadiene-2-carboxaldehydes (R = e.g., Me). The aldehyde positioned at C2 of the substrates enables iminium catalysis. The resulting enol intermediate then undergoes an intramolecular Michael reaction to give products with cyclopentane units.
The reaction gives a range of bicyclic and monocyclic products in moderate to excellent yields. The cascade can also be further extended by combining an α-methenylation with the oxy-Cope/Michael reaction. This combination expands the potential applications of the developed synthetic approach.
- An Organocatalytic Oxy-Cope/Michael Cascade Reaction,
Ryan R. G. Barrett, Donald A. Campbell, James L. Gleason,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.2c04269