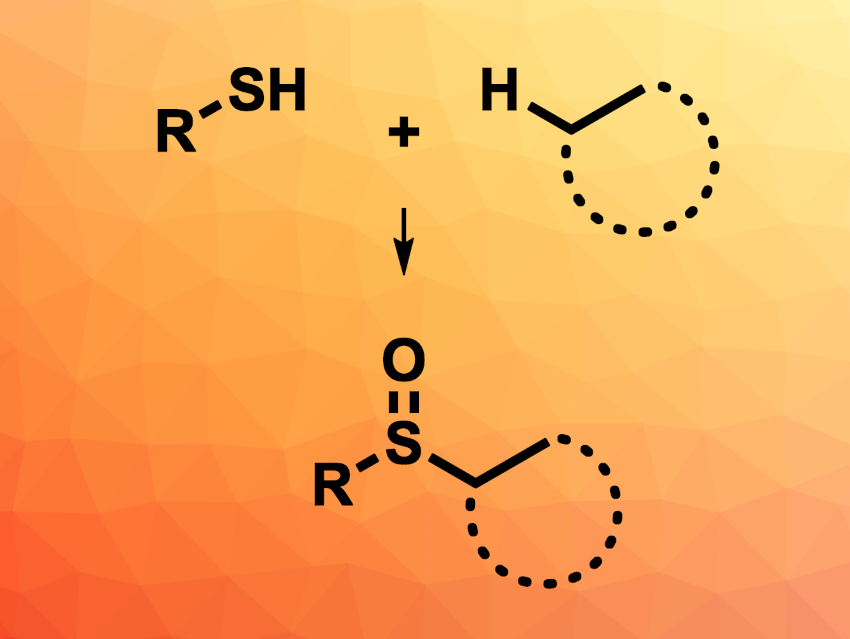
Sulfoxide groups are often found, e.g., in natural products or biologically active compounds. They can be synthesized, for example, via the oxidation of sulfides or from sulfenate precursors. However, these approaches require extra steps to prepare the necessary precursors. The use of thiols for the synthesis of sulfoxides via a direct C–S bond coupling could be a useful alternative.
Ya-Min Li, Kunming University of Science and Technology, China, and colleagues have developed a method for the oxidative dehydrogenative coupling of thiols with alkanes to give sulfoxides. The team reacted a variety of phenyl thiol derivatives with alkanes such as cyclopentane, cyclohexane, cyclooctane, or hexane in the presence of CuCl2 and benzoyl peroxide (BPO) as an oxidant, using CF3COOH (TFA) as an additive.
The desired sulfoxides were obtained in moderate to good yields. Both electron-rich and electron-poor phenyl thiols are suitable substrates. The non-aromatic hexane-1-thiol also underwent the reaction, but gave a lower yield. The team also performed the reaction on a gram scale, converting 4-methylbenzenethiol and cyclohexane into the desired sulfoxide in a yield of 62 %. The team proposes a mechanism that involves the homolysis of BPO, followed by hydrogen abstractions from the thiol and the alkane. The coupling of the resulting radicals is then followed by an oxidation to give the sulfoxide.
- Oxidative Dehydrogenative Coupling of Thiols with Alkanes for the Synthesis of Sulfoxides,
Yu-Jian Hou, Yi Li, Zhi-Wei Zhao, Tai-Gang Fan, Bo-Xun Sun, Xu-Nan Wang, Ya-Min Li,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.2c04238