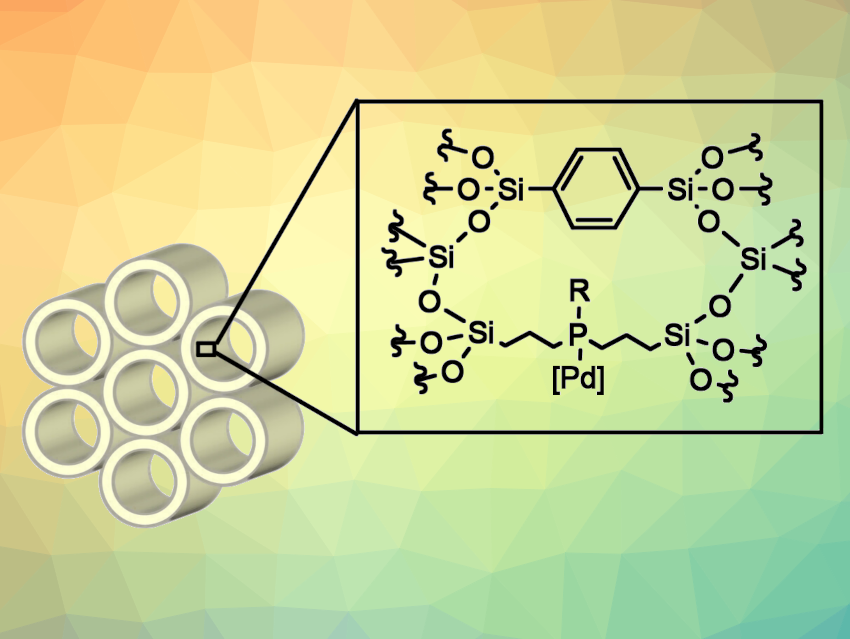
Periodic mesoporous organosilica (PMO) materials containing metal-coordinating units within the framework can be useful as catalyst supports. In these materials, the coordinating units can be placed in a siloxane network by hydrolysis and polycondensation of organosilane precursors in the presence of a surfactant as a structure-directing agent. Isolated catalytic sites can be established on the pore surface of such PMOs. However, examples with phosphine units as metal coordination sites are somewhat rare due to a complicated synthetic process for the corresponding organosilane precursors.
Yumiko Nakajima, National Institute of Advanced Science and Technology (AIST), Tsukuba, Japan, and Tokyo Institute of Technology, Japan, and colleagues have developed PMOs with phosphine-based coordinating units (pictured, R-P-PMO-TMS, R = Ph, tBu). The PMOs were synthesized using bifunctional compounds with alkoxysilyl and phosphino groups (i.e., bis[3-(triethoxysilyl)propyl]phenylphosphine borane or bis[3-(triethoxysilyl)propyl]-tert-butylphosphine borane) and 1,4-bis(triethoxysilyl)benzene by hydrolysis and polycondensation, followed by borane deprotection. Pd was then immobilized on the phosphine-based PMOs using palladium precursor complexes.
The team found that Pd(0) immobilized on the phosphine-PMOs is an efficient catalyst for Suzuki-Miyaura cross-coupling reactions of inactive aryl chlorides. The cross-coupling of sterically demanding substrates, which is difficult to achieve using conventional homogeneous catalyst systems, was successfully promoted.
- Suzuki‐Miyaura Cross‐Coupling Reaction Using Palladium Catalysts Supported on Phosphine Periodic Mesoporous Organosilica,
Kosuke Iizuka, Yoshifumi Maegawa, Yoshihiro Shimoyama, Kei Sakamoto, Natsuko Kayakiri, Yasutomo Goto, Yuki Naganawa, Shinji Tanaka, Masaru Yoshida, Shinji Inagaki, Yumiko Nakajima,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202303159