Nitroalkanes are useful building blocks in organic synthesis. The nitro group can be easily transformed into other functional groups, for example, by reduction to an amine. In nitroalkanes, the electron-withdrawing nitro group leads to an increased C–H-acidity in the α-position. Deprotonation at this position gives a nitronate anion, which can act as a nucleophile and form new C–C-bonds with carbon-based electrophiles.
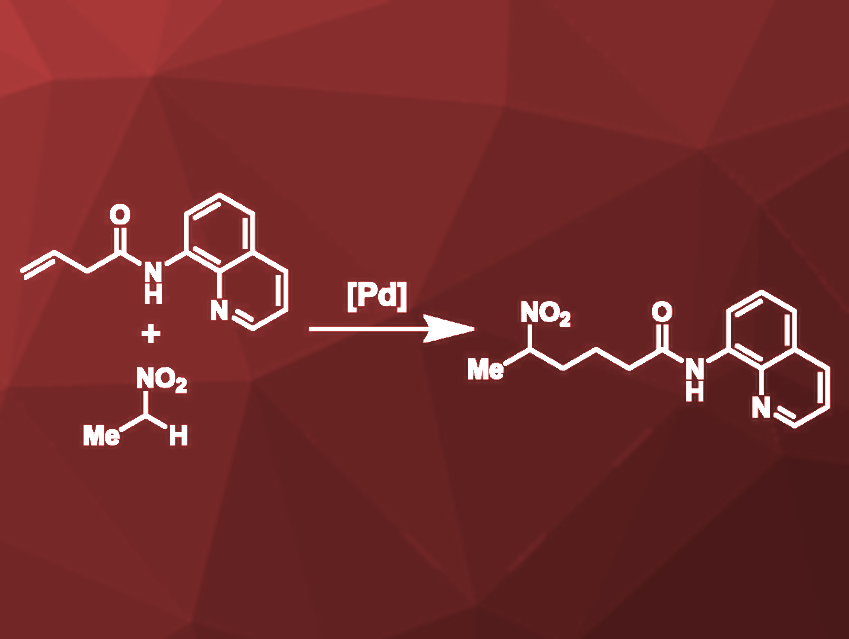
Peng Liu, University of Pittsburgh, PA, USA, Keary M. Engle, The Scripps Research Institute, La Jolla, CA, USA, and colleagues have developed a palladium-catalyzed addition of nitroalkanes to unactivated alkenes that are substituted by an 8-aminoquinoline amide (AQ) auxiliary group. The reaction was optimized for AQ-substituted 3-butenoic acid and an excess of nitroethane (pictured) which reacted to the target product in 83 % yield under the optimized conditions. PdI2 was used as a palladium source, sodium carbonate as a base, and hexafluoroisopropanol (HFIP) as the solvent.
The reaction was successfully extended to nitroalkanes and alkenes with more complex structures, as well as the intramolecular cyclization of substrates containing both alkene and nitroalkane groups in one molecule. An exemplary keto-nitroalkane selectively reacted at the α-position of the nitro group with a 78 % yield of the target product. In the proposed reaction mechanism, the alkene double bond is activated by the palladium. The resulting complex is attacked by the nitronate anion, leading to the formation of an alkyl palladacycle. A proton transfer then enables the release of the product and the regeneration of the catalyst.
- Catalytic Addition of Nitroalkanes to Unactivated Alkenes via Directed Carbopalladation,
Amit Kumar Simlandy, Warabhorn Rodphon, Turki M. Alturaifi, Binh Khanh Mai, Hui-Qi Ni, John A. Gurak, Peng Liu, Keary M. Engle,
ACS Catal. 2022.
https://doi.org/10.1021/acscatal.2c04557