Optically active sulfoxides are found in, e.g., pharmaceutical agents, natural products, chiral auxiliaries, and ligands for asymmetric catalysis. Joseph M. Ready, University of Texas Southwestern Medical Center, Dallas, USA, and colleagues investigated these compounds in the context of finding inhibitors of 15-prostaglandin dehydrogenase (15-PGDH). This enzyme metabolizes several prostaglandins and is a drug target. The large-scale synthesis of potent 15-PGDH inhibitors requires stereoselective access to sulfoxides.
The team first performed an enzymatic resolution of α-sulfinyl esters using lipoprotein lipase (pictured below). The resolution provides unreacted esters and the corresponding α-sulfinyl carboxylic acids in high enantiomeric purities. Both alkyl- and aryl-substituted products are accessible using this method. Subsequent decarboxylative halogenation, dihalogenation, trihalogenation, or cross-coupling reactions were then used to obtain functionalized sulfoxides.
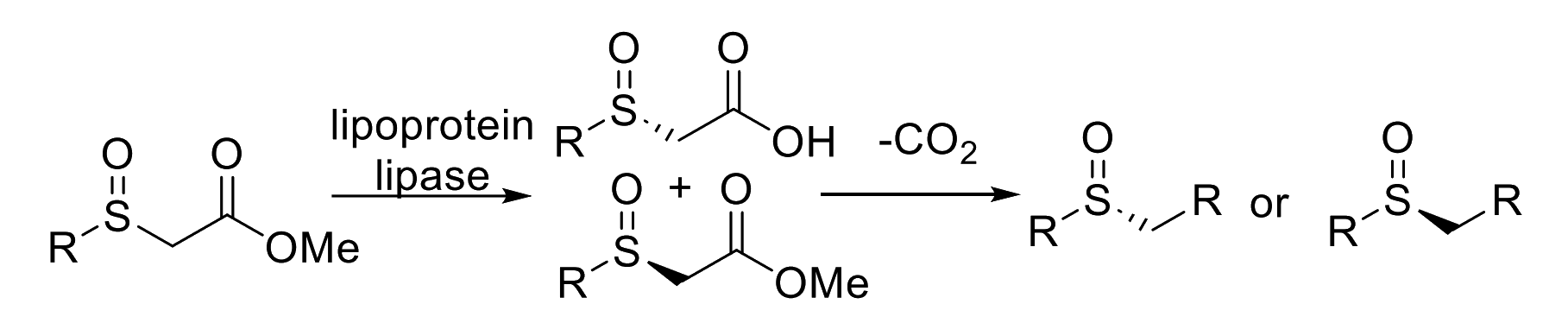
The researchers used the developed method for the asymmetric synthesis of a potent inhibitor of 15-prostaglandin dehydrogenase. The enzymatic resolution provides access to either enantiomeric form via an ester/acid interconversion. The decarboxylative functionalizations in the final step can proceed under retention of the enantiomeric purity, providing access to a wide range of optically active sulfoxides.
- Enzymatic Resolution and Decarboxylative Functionalization of α‐Sulfinyl Esters,
Suraksha Gahalawat, Yesu Addepalli, Stephen P Fink, Lakshmi Kasturi, Sanford D Markowitz, Joseph M. Ready,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202302996