Peptides are important products with a wide range of applications. Usually, synthetic peptides are prepared by synthesis in solution or via solid-phase peptide synthesis (SPPS). These existing methods, however, can have drawbacks when it comes to green chemistry aspects, requiring, e.g., large amounts of solvents, coupling reagents, or an excess of protected amino acids. Mechanochemical approaches could make peptide synthesis more environmentally friendly.
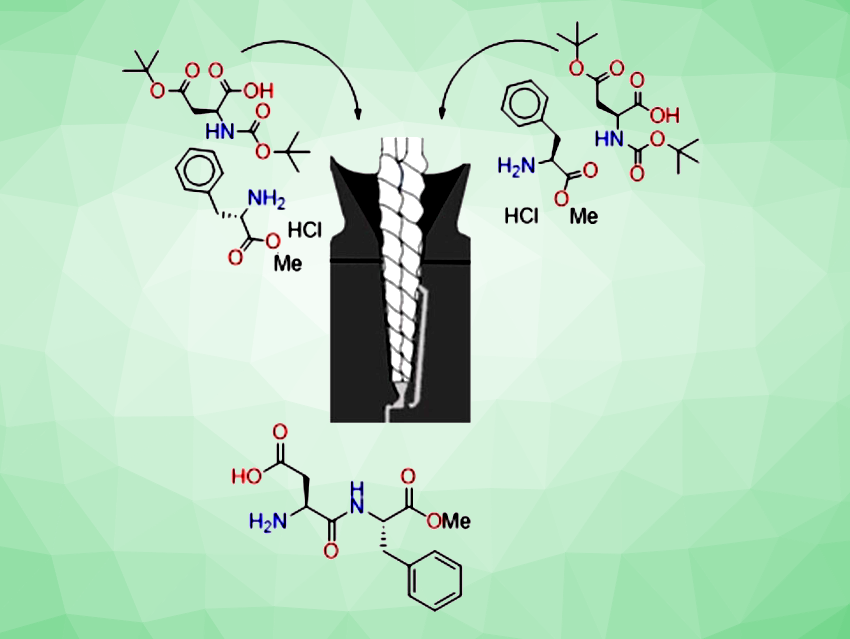
Tharwat Mohy El-Dine, Thomas-Xavier Métro, Frédéric Lamaty, University of Montpellier, France, and colleagues have developed a method for the synthesis of peptides from unactivated N-protected amino acids using a twin-screw extruder (TSE, pictured schematically). The extruder is a vertical parallel co-rotating twin screw with a conical shape, a 2 mL barrel volume, and a re-circulation channel facilitating both continuous and batch operation for extended reaction optimization
The team first optimized the reaction conditions using the coupling of Fmoc-His(Trt)-OH and S-phenylethylamine as a model reaction. In the presence of diisopropylcarbodiimide (DIC) and OxymaPure (ethyl cyano(hydroxyimino)acetate) as coupling reagents, and ethyl acetate (EtOAc) as liquid additive, the coupling product Fmoc-His(Trt)-S-phenylethylamide was obtained in 99 % isolated yield and with only 0.37 % epimerization after 4 min of re-circulation through the extruder at 40 °C.
The optimized method allows the preparation of a wide range of dipeptides and tripeptides with virtually no epimerization. The team applied this mechanochemistry approach to the synthesis of aspartame, a synthetic dipeptide, which was prepared in a continuous mode on a 0.215 mol scale. They obtained 56.2 g of pure aspartame (90 % yield).
- Synthesis of Peptides by Reactive Extrusion. Application to the Continuous and Solventless Preparation of Aspartame.,
Tharwat Mohy El‐Dine, Matthieu Lavayssiere, Hélène Adihou, Gilles Subra, Thomas‐Xavier Métro, Olivier Ludemann‐Hombourger, Frédéric Lamaty,
ChemistryEurope 2024.
https://doi.org/10.1002/ceur.202400007