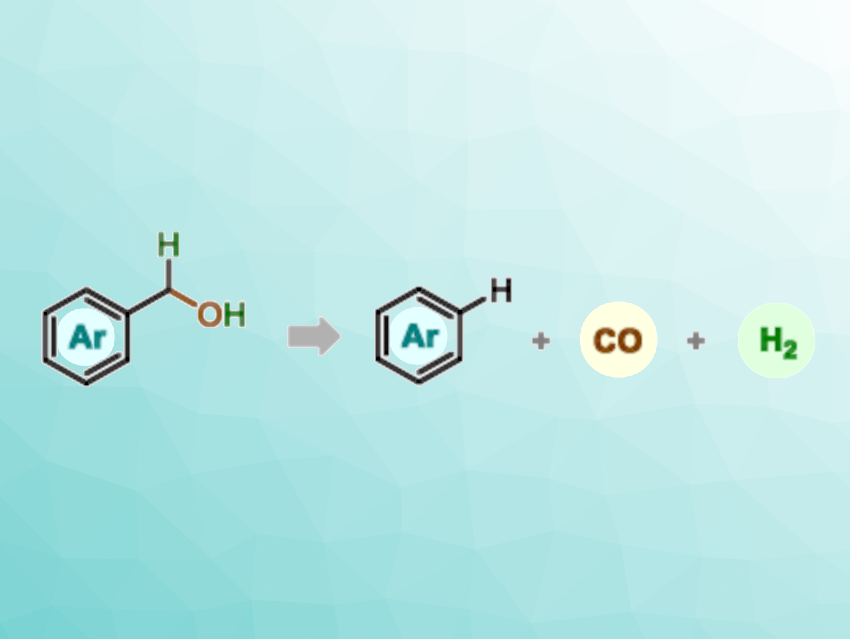
Hydroformylation reactions, i.e., functionalizations of alkenes with carbon monoxide and hydrogen (syngas) to generate the corresponding aldehydes, are well-established in industry. The reverse reaction, dehydroformylation, has remained relatively unexplored. In this reaction, instead of using syngas as a reactant, it is cleaved from alcohols or aldehydes in the presence of a metal catalyst. Using this approach, alkenes or arenes can be obtained from a vast pool of available starting materials in a straightforward manner.
Dehydroformylation protocols have typically relied on a combination of metal complexes and high temperatures. Photocatalytic approaches have been developed, as well, but the light-driven dehydroformylation of benzyl alcohols has remained elusive.
Burkhard König, University of Regensburg, Germany, and colleagues have combined photoinduced hydrogen atom transfer (HAT) and cobalt catalysis to develop a mild dehydroformylation sequence for the conversion of benzyl alcohols to arenes (pictured). The transformation proceeds from the alcohol to the corresponding aldehyde and the arene product via a stepwise radical pathway, with benzylic and acyl radicals as key intermediates and hydrogen and carbon monoxide as byproducts (pictured below). After a thorough screening, the team chose a combination of tetra-n-butylammonium decatungstate (TBADT) and cobaloxime pyridine chloride (COPC) as the optimal catalyst systems for the dehydroformylation of a wide array of benzyl alcohols under light irradiation at 385 nm.
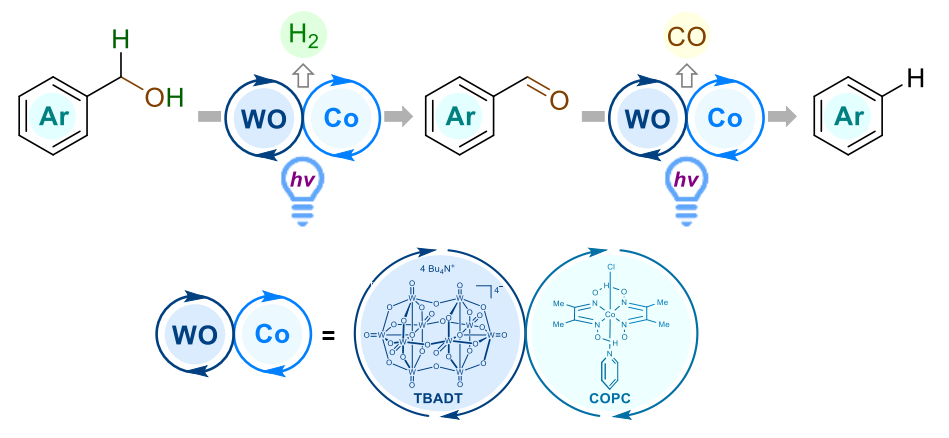
Different functional groups and substituents were found to be compatible with the system and remained intact upon dehydroformylation. Further development of dual HAT-cobalt systems could be useful, e.g., for the selective modification of natural product mixtures.
- Photocatalytic Dehydroformylation of Benzyl Alcohols to Arenes,
Daniel Kolb, Martin Morgenstern, Leon Ganser, Isabel Weidacher, Burkhard König,
ChemPhotoChem 2023.
https://doi.org/10.1002/cptc.202300167