Aminohalogenation reactions of alkenes are useful transformations in organic synthesis and give intermediates that can be reacted further. Using photochemistry for this type of reaction is attractive, but existing reactions can have drawbacks such as a need for expensive photocatalysts, a lack of selectivity, or limited atom economy.
Christoforos G. Kokotos, National and Kapodistrian University of Athens, Greece, and colleagues have developed a practical protocol for the aminochlorination of alkenes under mild photochemical conditions (pictured below). The strategy uses N-chlorosulfonamides as both nitrogen and chlorine source, and no external photocatalyst is required. The team optimized the reaction conditions using N-chloro-N-methyl-N-tosylamine and found that homolysis of the N–Cl bond could be achieved under LED irradiation in the UVA range (370 nm). The reactions were performed under an argon atmosphere at room temperature in CH2Cl2 as the solvent.
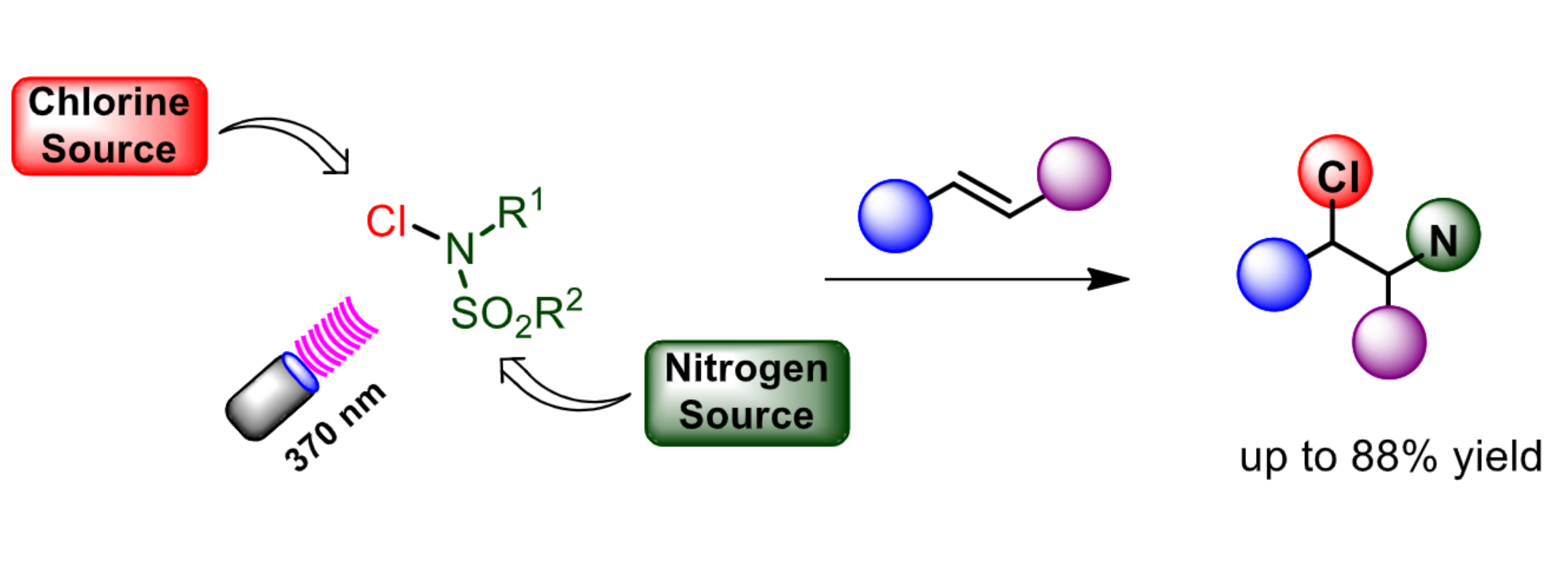
Using this approach, the team successfully converted a wide variety of olefins with a broad range of functional groups (28 examples, 18–88 % yield). The team proposes a reaction mechanism that involves homolysis of the nitrogen-chlorine bond of the N-chlorosulfonamide to give an N-centered radical and a chlorine radical. The nitrogen-based radical undergoes addition to the alkene. The resulting intermediate abstracts a chlorine radical from a second molecule of the N-chlorosulfonamide, giving the desired product and propagating the radical chain.
- Photochemical Aminochlorination of Alkenes Without the Use of an External Catalyst,
Constantinos T. Constantinou, Petros L. Gkizis, Olga Thomais G. Lagopanagiotopoulou, Elpida Skolia, Nikolaos F. Nikitas, Ierasia Triandafillidi, Christoforos G. Kokotos,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202301268