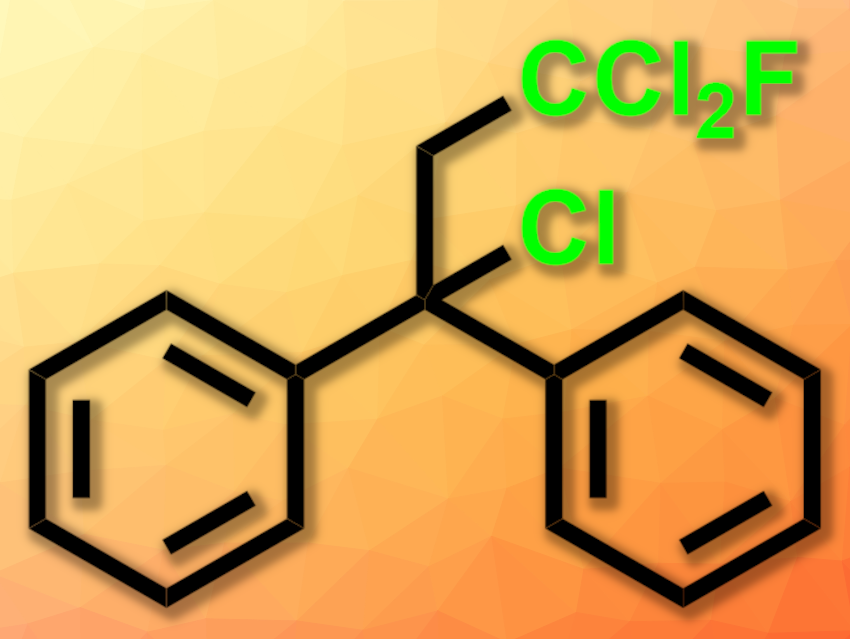
Fluorinated substituents play a key role in modern applied sciences, including medicinal chemistry, agrochemistry, and functional materials. In this context, mixed halogenated functional groups, such as the vicinal dichloro-fluorine group, are not widely used due to their costly and complex introduction. The high electronegativity of the fluorine substituent in the geminal position can activate the chlorine substituent for further useful chemical transformations.
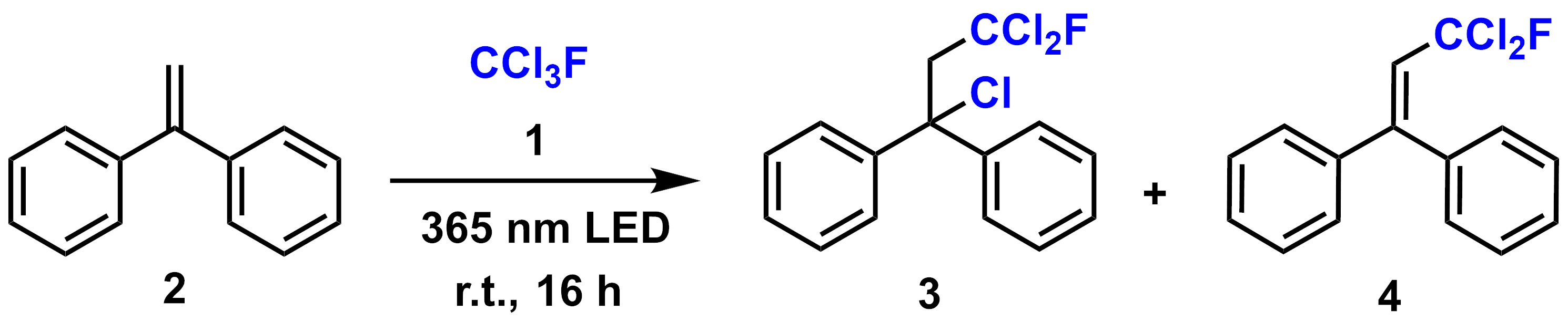
Hans-Achim Wagenknecht, Karlsruhe Institute of Technology (KIT), Germany, and colleagues have developed a simple method to activate CCl3F by photoredox catalysis and install the CCl2F radical on alkenes (pictured below). The organic photoredox catalyst N-phenylphenothiazine 1 activates the greenhouse gas and potent ozone-depleting molecule trichlorofluoromethane under photoirradiation. The CCl2F radical adds to substrate 2 in a Giese-type reaction, forming the dichlorofluoromethylated reaction product 3. Subsequent, often rapid, elimination of HCl gives the vinyl system 4.
The method tolerates electron-withdrawing functional groups such as nitrile ester carboxylic acid and amide groups. In contrast, electron-donating groups such as methoxy and dimethylamino groups were not competent substrates in the transformation.
Follow-Up Reactions
Subsequent reactions of functionalized geminal chlorofluorides to chlorofluorinated compounds show the versatility of the products. For example, starting from 4, amide 6 was obtained in 40 % yield by addition of silver salt and benzylamine (pictured below). In addition, by adding AgSbF6 and 1-methylindene to 4, the Friedel-Craft product 5-Z/E was formed in 95 % yield.
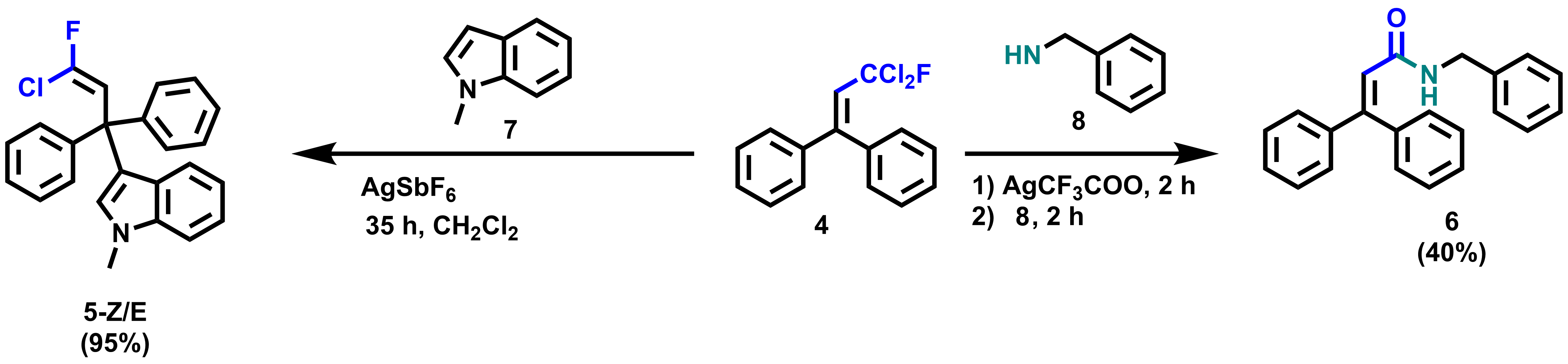
Overall, this method provides direct access to important dichlorofluoromethylated molecules by photoredox catalysis and provides versatility for further downstream transformations.
- Photoredox Catalytic Activation of Trichlorofluoromethane and Addition to Styrenes,
Desirée Steuernagel, David Rombach, Daniel Sack, Martin Koos, Burkhard Luy, Hans-Achim Wagenknecht,
ChemPhotoChem 2022.
https://doi.org/10.1002/cptc.202200289