|
Picture 52 |
Picture 53 |
Picture 54 |
 |
 |
 |
|
Picture by Konstantina Dodi Iokasti Flame |
Picture by Aleksandar Kondinski Chemistry is indeed an interplay of experiment and theory. Nowadays, with thousands of chemists working in front of computers, the definition of a lab goes beyond its original boundaries. |
Picture by Tina Michetti A redox reaction between KI and H2O2. |
|
Picture 55 |
Picture 56 |
Picture 57 |
 |
 |
 |
|
Picture by Tina Michetti Dry ice |
Picture by Subramanyan Namboodiri Varanakkottu SMART materials Spiropyran is a photosensitive smart material, which undergoes reversible structural change upon UV-Visible irradiation. The photograph shows the photochromatic properties of a water drop containing Spiropyran under different illumination conditions. |
Picture by Torsten John Made in Germany: The German Democratic Republic (GDR) and Western Germany united in a lab Glassware and its cleaning are the main part of a normal lab day for a chemist. You will find it “Made in Germany” all over the world, even in a lab in Australia, where this picture was taken. |
|
Picture 58 |
Picture 59 |
Picture 60 |
 |
 |
 |
|
Picture by Annika Buchheit and Mariano Grünebaum Formation of NaK alloy Formation of liquid NaK alloy by just placing a piece of sodium on potassium. |
Picture by Julia Bader Still life in purple Always look at your NMR tubes before washing them, you’ll be amazed at what you may find! |
Picture by Julia Bader Glass in motion? The beauty of a Vigreux column. |
|
Picture 61 |
Picture 62 |
Picture 63 |
 |
 |
 |
|
Picture by John Howse GE cyclotron, target filling station. Production 18F. |
Picture by Raquel De Francisco The amazing dancing floor A droplet of water bouncing on a superhydrophobic and light-emitting surface. To the left, a static water droplet looks on and wants to mimic it. The latter will become a awesome dancer very soon. This multifunctional coating, which combines superhydrophobicity and luminiscence, is based on polyfluorene and silica nanoparticles. It has been used to coat glass and celullose supports and maybe the basis for future highly hydrophobic electronics. |
Picture by Priyanka Patel Caffeine–salicylic acid co-crystal Dissolving caffeine and salicylic acid in acetonitrile and leaving the solution overnight gives this type of co-crystal the next morning.
|
|
Picture 64 |
Picture 65 |
Picture 66 |
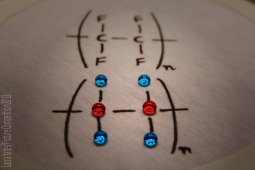 |
 |
 |
|
Picture by Laura Yates Water bead ‘molymod’ A ‘molymod’ style structure of a fluorocarbon made by using beads of colored water to represent the carbon and fluorine atoms. This has been carried out on a piece of fabric which has been treated with a fluorocarbon rendering it water repellent. |
Picture by Tobius Dörr The crystallization of “syncarpinsäure” |
Picture by Mohsen Ahmadi My synthesized materials, made with minimal facilities!
|
|
Picture 67 |
Picture 68 |
Picture 69 |
 |
 |
 |
|
Picture by Zahra Nasri Work on the glutamate dehydrogenase (GDH) enzyme |
Picture by Zahra Nasri My electrochemical cell |
Picture by Zahra Nasri My great experiment (I guess!) |
|
Picture 70 |
Picture 71 |
Picture 72 |
 |
 |
 |
|
Picture by Johannes Thomé This picture was taken while a middle school class had their first experience with chemistry. The children had a lot to be astonished about. |
Picture by Christoph Göbel Silica Stalagmite |
Picture by Melanie Schnabel “Green” Chemistry |
|
Picture 73 |
Picture 74 |
Picture 75 |
 |
 |
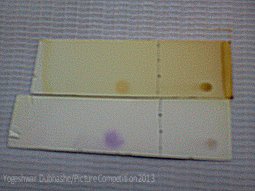 |
|
Picture by Melanie Schnabel Violent Violet An oxidation reaction with potassium permanganate was too violent. |
Picture by Melanie Schnabel Checking the Vacuum |
Picture by Yogeshwar Dubhashe Thin layer Chromatography (TLC) of Indole. |
|
Picture 76 |
Picture 77 |
Picture 78 |
 |
 |
 |
|
Picture by Maysoon Alhafez and Rahaf Alhalabi Cobalt Cobalt chloride as a solid and in solution. |
Picture by Maysoon Alhafez and Rahaf Alhalabi Colorful Salt Solutions |
Picture by Eva Mutoro Happy Lab |
|
Picture 79 |
Picture 80 |
Picture 81 |
 |
 |
 |
|
Picture by Yazan Nader Experience in Analytical Chemistry Chloroform with iodine |
Picture by Lena Thomé Colorful Test Tubes |
Picture by Yazan Nader |
|
Picture 82 |
Picture 83 |
Picture 84 |
 |
 |
 |
|
Picture by Ruba Jarrar Simple Distillation |
Picture by Benjamin Schneider Blue Bubbles |
Picture by Benjamin Schneider Mixing in Water |
|
Picture 85 |
Picture 86 |
Picture 87 |
 |
 |
 |
|
Picture by Lena Thomé Ionic Crystal Tower |
Picture by Hendrik Pöpke Smoking Liquids Gas phase reaction of hydrochloric acid and ammonia |
Picture by Eva Mutoro Chromatography |
|
Picture 88 |
Picture 89 |
Picture 90 |
 |
 |
 |
|
Picture by Ansar Nazzal Molecular Fluorescence Spectroscopy Measuring emission and excitation |
Picture by Oana Fronoiu Acetone and Liquid Nitrogen |
Picture by Oana Fronoiu Acetone and Liquid Nitrogen |
|
Picture 91 |
Picture 92 |
Picture 93 |
 |
 |
 |
|
Picture by Oana Fronoiu |
Picture by Oana Fronoiu Chemiluminescence |
Picture by Oana Fronoiu Controlled Release of Drugs |
|
Picture 94 |
Picture 95 |
Picture 96 |
 |
 |
 |
|
Picture by Oana Fronoiu Red Cabbage – pH Indicator |
Picture by Oana Fronoiu Vintage Lab |
Picture by Lidia Minza |
|
Picture 97 |
Picture 98 |
Picture 99 |
 |
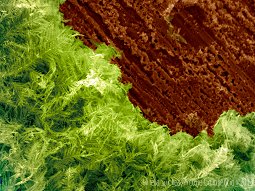 |
 |
|
Picture by Lidia Minza Mess in the Lab! |
Picture by Mirela Suchea Ancient beauty A digitally colored scanning electron image of crystalline silver grown onto copper in a silver nitrate solution to give a “silver-tree” or, going back to the times of the alchemists, “Arbor Dianæ”. |
Picture by Farah Brangakgi Fehling Solution Boiling Fehling 1 and Fehling 2 solutions containing sugar. A brown color precipitate should appear. |
|
Picture 100 |
Picture 101 |
Picture 102 |
 |
 |
 |
|
Picture by Charles Bou Nader Laboratory Fumehood for Homogeneous Catalysis Experiments A sudden idea related to a mechanism emerges during an experiment. Unfortunately, you have nothing to write on except “the hood”. Sometimes inspiration gets the best of you and there is no time to waste! The beauty of chemistry comes from our creativity and imagination, allowing us to push even further the limits of our knowledge and therefore our curiosity. |
Picture by Ruba Jarrar Simple Filtration |
Picture by Zahra Rashedi Recovery of Chemical Wastes
|