Synthesizing compounds with multiple bonds between boron and another element can be challenging, especially with heavier elements such as tin. Compounds with an Sn=B double bond, i.e., stannaborenes, have remained elusive so far, while some examples with E=B bonds are known for the lighter elements Si and Ge.
Christian P. Sindlinger, University of Stuttgart, Germany, Lars Wesemann, University of Tübingen, Germany, and colleagues have synthesized compounds with Sn=B units, i.e., a stannaborenyl anion and a stannaborenium cation. The team started from a stannylene Lewis pair with a phosphine Lewis base, which was reacted with BBr3·SMe2 via an oxidative addition at the tin atom.
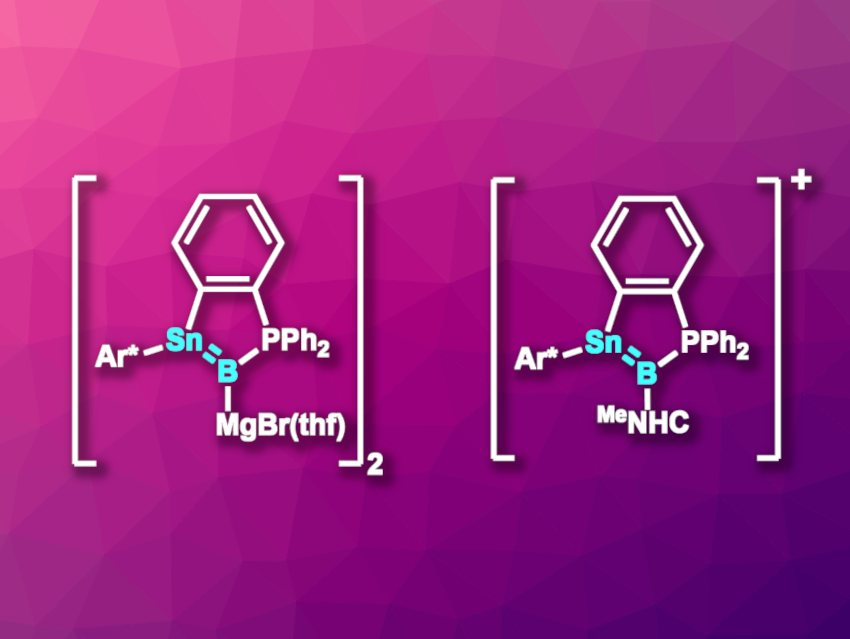
The resulting boron-containing product was reduced using excess Mg/anthracene/dibromoethane to give a stannaborenyl compound. The product crystallizes as a dimeric magnesium bromide salt (pictured above on the left, Ar* = C6H3(2,6-Trip)2, Trip = 2,4,6-C6H2iPr3). The compound has an Sn–B bond length that is shorter than typical single bonds between boron and tin, and DFT calculations support the existence of a Sn–B π-bond.
The researchers also subjected the addition product of boron tribromide and the stannylene-phosphine Lewis pair to hydride substitution. This was followed by a reaction with MeNHC (MeNHC = 1,3,4,5-tetramethylimidazol-2-ylidene) under HBr elimination to give a carbene- and phosphine-stabilized stannyl-borylene, i.e., [o-C6H4(Ar*BrSn-B{PPh2}{MeNHC})]. The remaining bromide was removed using Li[Al(OC{CF3}3)4] or Na[BArF4], i.e., salts with weakly coordinating anions. Thus, the team obtained a stannaborenium cation (pictured above on the right).
- Authentic Sn═B-Double Bonds in Polar Stannaborene Derivatives,
Magda Zweigart, Klaus Eichele, Hartmut Schubert, Christian P. Sindlinger, Lars Wesemann,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c03744