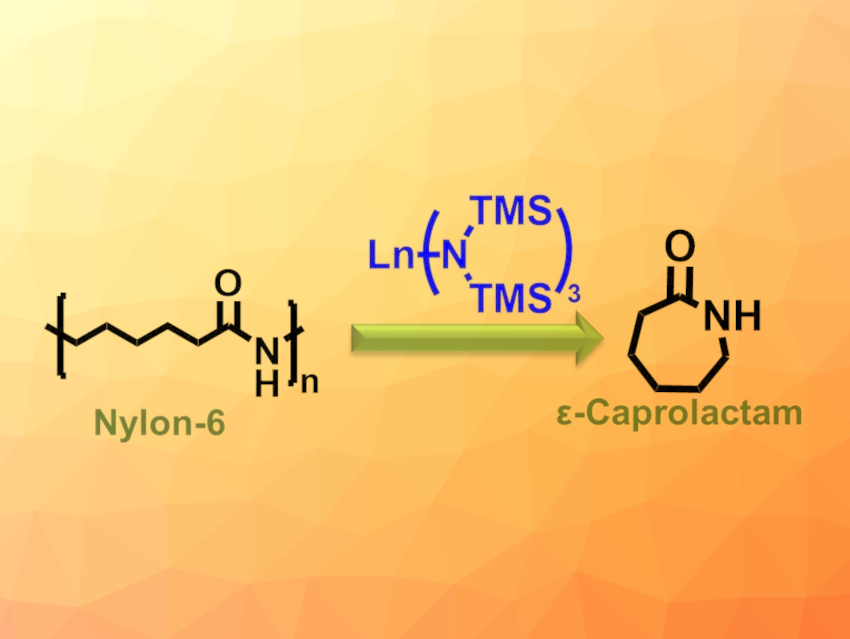
Nylon-6 is a tough, non-biodegradable plastic that cannot be recycled by conventional methods. A new way has been introduced by Tobin J. Marks, Yosi Kratish, Northwestern University, Evanston, IL, USA, and colleagues: With an easily accessible lanthanum trisamido catalyst, Nylon-6 can be depolymerized highly selectively, nearly quantitatively, with no solvent, and at moderate temperatures to recover the monomer, ε-caprolactam. The monomers are removed sequentially from one polymer end, just like unthreading pearls from a chain.
Nylon Waste
Nylon is the fabric from which stockings are made. It is also the material of choice for many applications in areas including automobile manufacturing, packaging, infrastructure, textiles, and fishing. Its advantageous properties, such as elasticity, chemical resistance, high tensile strength, and high abrasion resistance get in the way of its biodegradability. Abandoned nylon fishing nets, for example, account for about 10 % of the plastic waste in the oceans.
Industrially, the variant Nylon-6 is made through a ring-opening polymerization of ε-caprolactam on a scale of 5 million tons a year. The market volume is projected to reach 21.5 billion dollars by 2026. The heaps of trash are growing correspondingly, increasing the danger to the environment and our health. In addition, the production of Nylon-6 has a large CO2 footprint. The monomer, ε-caprolactam, is made from fossil-based raw materials in a costly, multistep process. Its reclamation would conserve resources and save on production costs and energy. There is, thus, a high demand for a circular economy for Nylon-6.
Chemical Recycling
While recycling for some other plastics is slowly ramping up, Nylon-6 is very difficult to recycle. Melting it to give it a new form is not possible because it partially decomposes at the high temperatures required. Burning it for energy production is also not possible because it forms toxic compounds like hydrogen cyanide. Previous methods of chemical recycling have proven to be too complex and ineffective or require problematic chemicals.
The team has developed a new, efficient catalytic process for recycling Nylon-6. Nylon-6 is depolymerized to ε-caprolactam with over 95 % selectivity and over 90 % yield—without solvents or toxic chemicals and at the comparably mild temperature of 240 °C. Admixtures of polyethylene, polypropylene, or polyethylene terephthalate do not interfere.
The team’s success rests on a catalyst based on commercially available trisamido complexes of rare earth metals. A lanthanum complex demonstrated the highest catalytic activity. Experimental data and computations suggest a novel mechanism for the reaction. In the first step, a hydrogen ion is removed from a terminal amide N–H bond and the catalyst is covalently bound to the polymer. Subsequently, the ε-caprolactam units are split off the end of the chain one by one in a backbiting process.
- Selective Lanthanide‐Organic Catalyzed Depolymerization of Nylon‐6 to ϵ‐Caprolactam,
Lukas Wursthorn, Kristen Beckett, Jacob O. Rothbaum, Robin M. Cywar, Clarissa Lincoln, Yosi Kratish, Tobin J. Marks,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202212543