Nigeria, the most populous country in Africa, is also the most malaria-affected country in the world, accounting for 32 % of global malaria deaths, according to the WHO. Treating malaria costs Nigeria’s economy $1.1 billion annually. Nigeria has provisionally approved the R21/Matrix-M malaria vaccine, which was designed and developed by scientists at the University of Oxford,UK. This approval came less than a week after Ghana approved the vaccine, making it the first regulatory clearance for the R21/Matrix-M malaria vaccine for use in any country.
The R21/Matrix-M vaccine has undergone clinical trials in the UK, Thailand, and several African countries, including an ongoing phase III trial in Burkina Faso, Kenya, Mali, and Tanzania, which has enrolled 4,800 children. The R21/Matrix-M vaccine is intended to prevent clinical malaria in children aged five to 36 months, who are at the highest risk of death from malaria. The vaccine has a storage temperature of 2–8 °C. The primary immunization regimen of the vaccine candidate consists of three doses and has shown more than 75 % efficacy in African children for a period of 12 months. A fourth booster dose was administered in the same group of children 12 months after the primary vaccination.
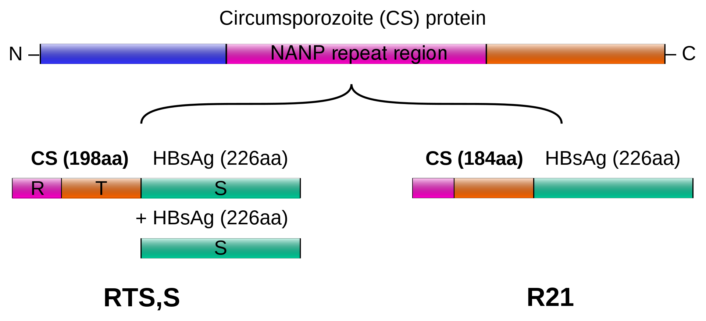
The currently only approved vaccine for malaria, Mosquirix (RTS,S/AS01), developed over 30 years by GlaxoSmithKline (GSK), UK, provides inadequate protection against the tropical disease. One year after administration of four doses, it had shown an efficacy of 56 %. However, three years later, its effectiveness had dropped to 36 %.
R21/Matrix-M is an advancement of RTS,S, combined with a more potent adjuvant, the saponin adjuvant (Matrix-M) from the company Novavax. Saponin is an extract from the bark of the soapberry tree. The technology has been successfully used in Novavax’s COVID-19 vaccine and is a key component of other development-stage vaccines. The Matrix-M adjuvant stimulates the entry of antigen-presenting cells at the injection site and enhances antigen presentation in local lymph nodes.
Like RTS,S, the vaccine contains a protein of the parasite (circumsporozoite) as well as a carrier protein derived from the hepatitis B virus. However, R21 uses a lower amount of the hepatitis protein, which is intended to allow the human immune system to concentrate more on the formation of antibodies against malaria.
Creative Commons Attribution-Share Alike 4.0 International, Julius Senegal
For many decades, research has been conducted on a vaccine against malaria. One hurdle is that the malaria parasite is a much more complex organism than a virus and also undergoes a complicated life cycle with several different stages, providing many potential targets for a vaccine. The vaccine targets the point where the parasite has just entered the human body and the number of these single-celled organisms is not yet so great.
[1] Mehreen S. Datoo, Hamtandi Magloire Natama, Athanase Somé, Duncan Bellamy, Ousmane Traoré, Toussaint Rouamba, Marc Christian Tahita, N Félix André Ido, Prisca Yameogo, Daniel Valia, Aida Millogo, Florence Ouedraogo, Rachidatou Soma, Seydou Sawadogo, Faizatou Sorgho, Karim Derra, Eli Rouamba, Fernando Ramos-Lopez, Matthew Cairns, Samuel Provstgaard-Morys, Jeremy Aboagye, Alison Lawrie, Rachel Roberts, Innocent Valéa, Hermann Sorgho, Nicola Williams, Gregory Glenn, Louis Fries, Jenny Reimer, Katie J Ewer, Umesh Shaligram, Adrian V S Hill, Halidou Tinto, Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years’ follow-up in children in Burkina Faso: a phase 1/2b randomised controlled trial, The Lancet 2022. https://doi.org/10.1016/S1473-3099(22)00442-X
[2] The study is registered with ClinicalTrials.gov (NCT03896724; Safety, Immunogenicity and Efficacy of R21 Matrix-M in 5-17 Month Old Children in Nanoro, Burkina Faso).
[3] World malaria report 2022, World Health Organization, 2022. ISBN 978-92-4-006489-8
[4] Christian Kretschmer, Neuer Malaria-Impfstoff überzeugt in klinischen Studien, Gelbe Liste 16.09.2022. (accessed April 19, 2023)
[5] R21 Malaria Investigational Vaccine, Novavax (accessed April 19, 2023)