For Halloween, we always like to find a fun connection to chemistry. In past years, we at ChemistryViews have created quizzes, shared pumpkin pie recipes, and recorded videos of bubbling pumpkins.
This time, we are focusing on molecules:
- Those named after the pumpkin (or, more precisely, Cucurbita, Latin for “gourd”, a genus of herbaceous fruits in the gourd family, Cucurbitaceae, also known as cucurbits or cucurbi) and
- Those commonly found in pumpkins—besides water, of course. 😉
1 Cucurbiturils
🏷️ Name
Derived from the Latin word Cucurbita, as they look similar to a pumpkin or a barrel.
⚛️ Structure
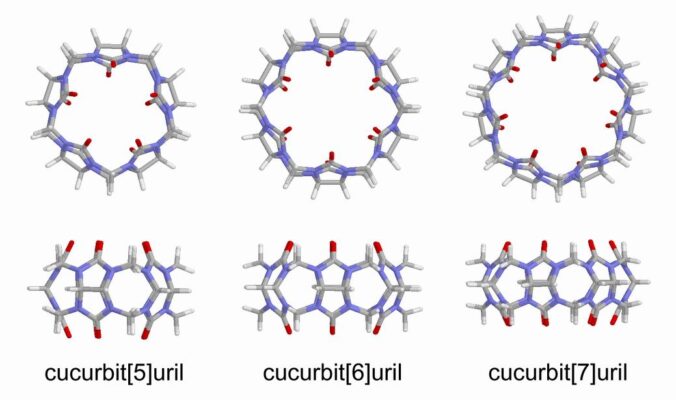
Macrocyclic, pumpkin-shaped, molecules composed of glycoluril units (=C₄H₂N₄O₂=) linked by methylene groups, forming a rigid, symmetrical cavity capable of encapsulating guest molecules.
Discovery & Synthesis of CB6
Cucurbituril (cucurbit[6]uril, or CB6) is a hexameric macrocyclic compound self-assembled from an acid-
catalyzed condensation reaction of glycoluril and formaldehyde. It was first synthesized in 1905 by Robert Behrend [1]. However, its chemical nature and structure had been unknown until 1981, when full characterization was reported by W. A. Freeman, W. L. Mock, and N.-Y. Shih [2].
CB6 has a cavity of ∼5.5 Å diameter, accessible from the exterior by two carbonyl-laced portals of ∼4 Å
diameter. The cavity has a height ~9.1 Å.
CBn Variants
Its variants, denoted cucurbit[n]uril (where n = 5–8, 10, etc.) and often abbreviated CBn, differ by the number of glycoluril units, influencing cavity size and binding affinity. CB5, CB7, and CB8 were discovered and isolated by Kim Kimoon, Pohang University and Science and Technology, South Korea, and colleagues in 2000 [3].
CC BY-SA 3.0
⚙️ Function
As mentioned above, cucurbiturils can encapsulate other molecules within their inner cavity. They show exceptional host–guest chemistry, forming highly stable inclusion complexes via hydrophobic and ion–dipole interactions. These properties make them valuable in supramolecular chemistry, drug delivery, and molecular recognition research.
Because CB[n] binding is strong, selective, and regularly reversible, they have been used to build sensors (for ions, small molecules), separation systems (e.g., removal of pollutants or radioactive species), and stimuli‐responsive catalytic systems.
The binding is largely driven by hydrophobic interactions inside the cavity plus cation–dipole and ion–dipole interactions at the carbonyl‐lined portals of the macrocycle. Guest ingress/egress, binding kinetics, and thermodynamics are sensitive to guest size, charge, portal interactions, salt concentration, pH.
Cucurbiturils have also been used as the macrocycles component of a rotaxane.
|
|
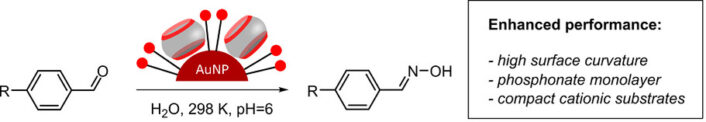
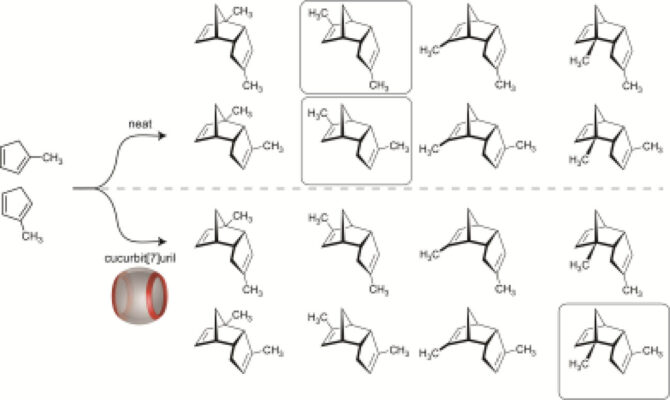
🧪 Recent Research Examples from Chemistry EuropeChemical Functionalization: Anthony I. Day, University of New South Wales Canberra, Australia, and colleagues have added a stable tetrahydrothiopheno group to glycoluril units, then tested ways to chemically modify these units and successfully applied the method to cucurbituril molecules CB[7] and CB[8]. The tetrahydrothiopheno group is robust under harsh reaction conditions and can be selectively reacted, allowing functionalized cucurbiturils that were previously difficult to modify. This method opens the door to creating new cucurbituril derivatives with tailored properties for drug delivery and other biomedical applications, expanding the toolbox for designing functional molecular hosts. [4] Catalysis Boost: Paola Posocco, University of Trieste, Italy, and Volodymyr Sashuk, Polish Academy of Sciences, Warsaw, and colleagues have built “suprazymes” by embedding cucurbit[7]uril macrocycles into negatively charged gold nanoparticle coatings and tested how substrate charge, size, and nanoparticle features affect catalysis. The combination of nanoparticle structure, macrocycle binding, and substrate design controls reaction efficiency, with a phosphonate-terminated monolayer boosting catalytic rates over 2400-fold compared to no catalyst. The researchers say that their work provides design rules for creating highly efficient, enzyme-like nanoparticle catalysts, useful for green chemistry, drug synthesis, and molecular engineering. [5] Selective Acceleration: Khaleel I. Assaf, Al-Balqa Applied University, Jordan, Werner M. Nau, Constructor University, Bremen, Germany, and colleagues have studied how cucurbit[7]uril (CB7) macrocycles affect the dimerization of methylcyclopentadiene in water, focusing on both reaction speed and which product forms. CB7 speeds up the reaction 104-fold and directs it to produce a single product that is normally minor, because the molecules fit optimally inside the macrocycle’s cavity. This shows that rigid macrocycles can control both rate and product selectivity, offering a way to mimic enzyme-like precision in synthetic reactions. [6] |
2 Molecules Found in Pumpkins
🍬 Carbohydrates
Sugars and structural polysaccharides such as pectin are major components of the flesh and peel.
🟠 Carotenoids
Pigments responsible for the pumpkin’s bright orange color. These molecules absorb blue light and reflect the remaining wavelengths, which we perceive as orange. Pumpkin pulp, peel and seeds are rich in carotenoids, especially β carotene (pictured below), which gives the orange/yellow color and serves as a provitamin A precursor.
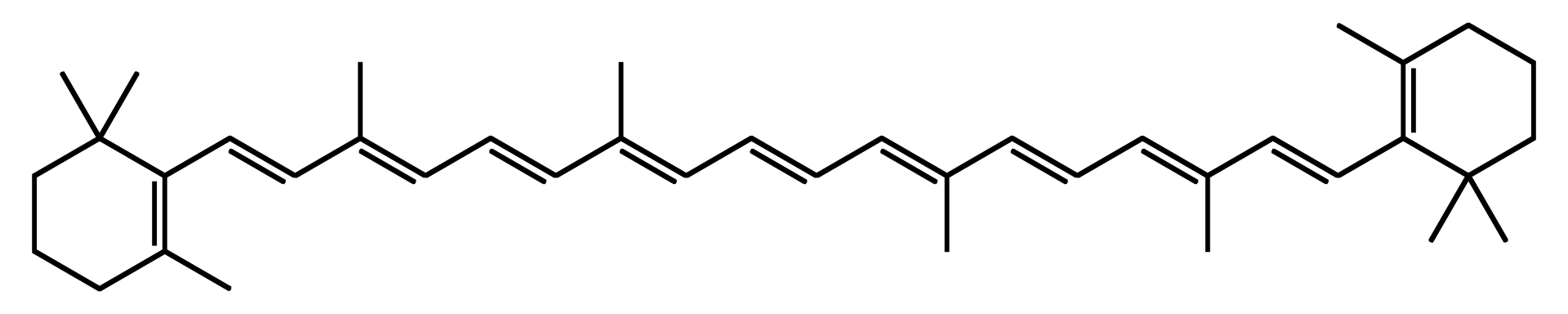
🪔 Plant Oils
Pumpkin seeds contain fatty oils rich in unsaturated fatty acids, including oleic, linoleic, and palmitic acid, as well as Δ⁷-sterols (avenasterol, spinasterol) and Δ⁵-sterols (β-sitosterol (pictured below), stigmasterol).
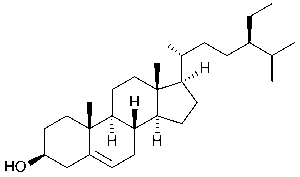
💊 Vitamins & Antioxidants
Pumpkins are a good source of vitamins, particularly provitamin A (β-carotene).
They also contain polyphenols (phenolic acids, flavonoids) and tocopherols (vitamin E variants) that contribute antioxidant activity.
😖 Cucurbitacins
Bitter tetracyclic terpenes with steroidal structures found in some pumpkins—members of the Cucurbitaceae family—that may cause poisoning if consumed in excessive amounts. They are thought to play a defensive role in plants against herbivores.
References
[1] Robert Behrend, Eberhard Meyer, Franz Rusche, I. Ueber Condensationsproducte aus Glycoluril und Formaldehyd, Justus Liebigs Annalen der Chemie 1905. https://doi.org/10.1002/jlac.19053390102
[2] W. A. Freeman, W. L. Mock, N. Y. Shih, Cucurbituril, J. Am. Chem. Soc. 1981, 103(24), 7367–7368
https://doi.org/10.1021/ja00414a070
[3] Jaheon Kim, In-Sun Jung, Soo-Young Kim, Eunsung Lee, Jin-Koo Kang, Shigeru Sakamoto, Kentaro Yamaguchi, Kimoon Kim, New Cucurbituril Homologues: Syntheses, Isolation, Characterization, and X-ray Crystal Structures of Cucurbit[n]uril (n = 5, 7, and 8), J. Am. Chem. Soc. 2000, 122(3), 540–541. https://doi.org/10.1021/ja993376p
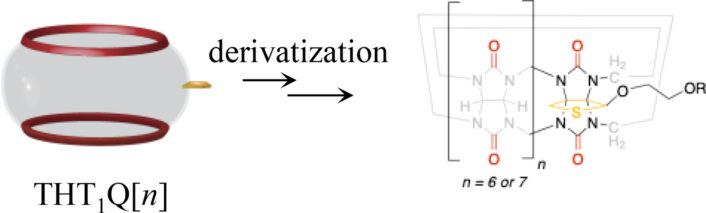
[4] Ahmed Abdulrahman, Mohamed M. S. Hamoud, Vijaybabu Mandadapu, Yi Zhao, Minghua Chen, Anthony I. Day, An Approach to Functionalization of Cucurbit[n]uril Through a Tetrahydrothiopheno, Eur. J. Org. Chem. 2025. https://doi.org/10.1002/ejoc.202401425
[5] Pavlo Hyziuk, Matteo Flaibani, Paola Posocco, Volodymyr Sashuk, Cucurbituril–Gold Nanoparticle Assemblies for Aqueous Oxime Formation Catalysis, ChemCatChem 2025. https://doi.org/10.1002/cctc.202500787
[6] Khaleel I. Assaf, Foad N. Tehrani, Guillermo E. Quintero, Robert Hein, Margarita E. Aliaga, Werner M. Nau, Regioselective Dimerization of Methylcyclopentadiene inside Cucurbit[7]uril, Chem. Eur. J. 2024. https://doi.org/10.1002/chem.202403964
Also of Interest

A perfect kitchen experiment for young kids and fun for all ages!

Pumpkins, sweets, spiders, champagne, … there is chemistry involved everywhere

We wish you a very Happy Halloween with this science-related quiz

Pumpkins contain healthy compounds, so why not bake a pumpkin pie?