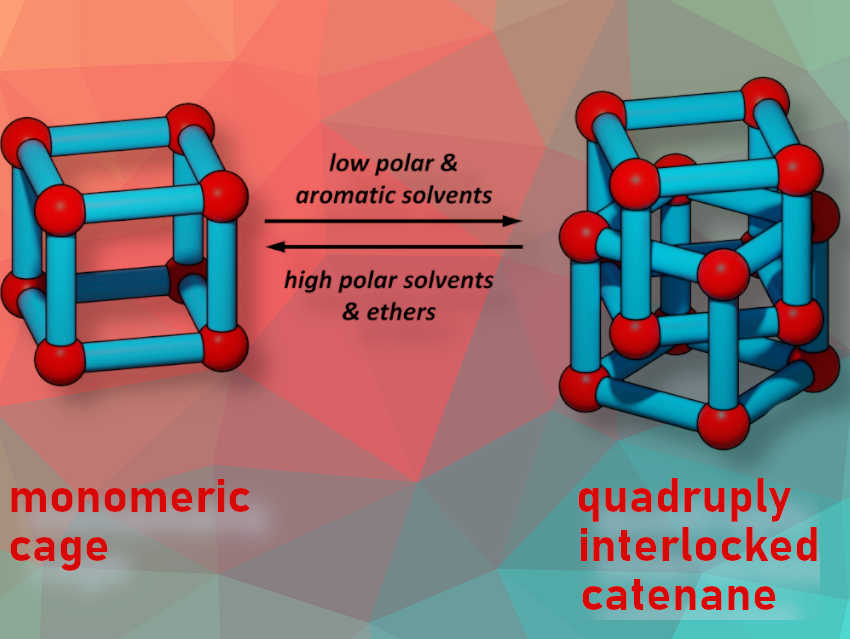
A catenane is a molecular architecture consisting of two or more interlocked macrocycles. So far, there are only a few examples of catenated organic cage molecules, and controlling the catenation process in solution is still challenging.
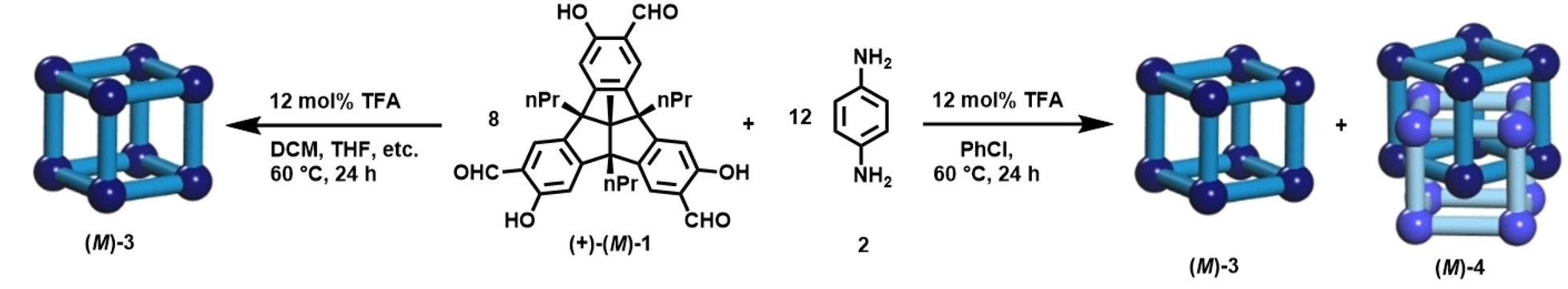
Michael Mastalerz and colleagues, Ruprecht-Karls-Universität Heidelberg, Germany, have synthesized the quadruply interlocked catenane (M)-4 of giant chiral [8+12] salicylimine cubes (M)-3. The team found that the acid-catalysed reaction between enantiomerically pure tribenzotriquinacene (TBTQ)-salicylaldehyde (+)-(M)-1 and the amine 2 in various solvents (CH2Cl2, CDCl3, tetrachloroethane, THF, 1,4-dioxane, DMSO) gives the pure monomeric cubic [8+12]-giant cage (M)-3 (pictured below left). In chlorobenzene, both the [8+12] cage (M)-3 and a [16+24] cage (M)-4 are formed (pictured below right).
The team discovered an inverse correlation between solvent polarities and catenane/single cage ratios. The formation of catenane (M)-4 was favored in less polar solvents. In contrast, highly polar solvents like nitrobenzene only produced the single cage (M)-3, and a mixture of single cage (M)-3 and catenane (M)-4 was formed in non-polar aliphatic solvents like pentachloroethane and chlorocyclohexane ((M)-4/(M)-3 = 92 : 8 in PhMe). This process is reversible. For example, a mixture with catenane (M)-4 as main component can be transferred to the cage by replacing toluene as solvent with one in which the cage is the dominant component, such as CD2Cl2. The researchers identified weak hydrogen bonding as a central driving force for the catenation process, which also explains the influence of the solvent.
The catenane (M)-4 was unambiguously characterized by single crystal X-ray diffraction as a dimeric, quadruply interlocked catenane, making it one of the largest organic cage catenanes characterized by this method and the largest chiral and imine cage catenane.
By using partially deuterated single cages in scrambling experiments, the team found that the catenation occurs through mechanical interlocking of preformed single cages, during which some of the 1,4-phenylenediamine linker units dissociate from the cage, while the TBTQ-corner units remain intact.
According to the researchers, their discoveries may pave the way for larger and more complex purely organic interlocked structures, although it is just one more piece of the puzzle in understanding and controlling catenation events by weak interactions.
- Solvent-Controlled Quadruple Catenation of Giant Chiral [8+12] Salicylimine Cubes Driven by Weak Hydrogen Bonding,
Philippe Wagner, Frank Rominger, Jürgen H. Gross, Michael Mastalerz,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202217251