Upon light excitation, electron donor–acceptor (EDA) complexes can generate radical intermediates, which can be useful in organic synthesis. Such EDA-complex-mediated approaches have an advantage over photoredox catalysis processes because they do not need a separate photocatalyst, toxic reagents, or transition metals. EDA complexes can, for example be used for the cyclization of 1,n-enynes (n = 5,6,7), giving five- and six-membered heterocyclic molecule.
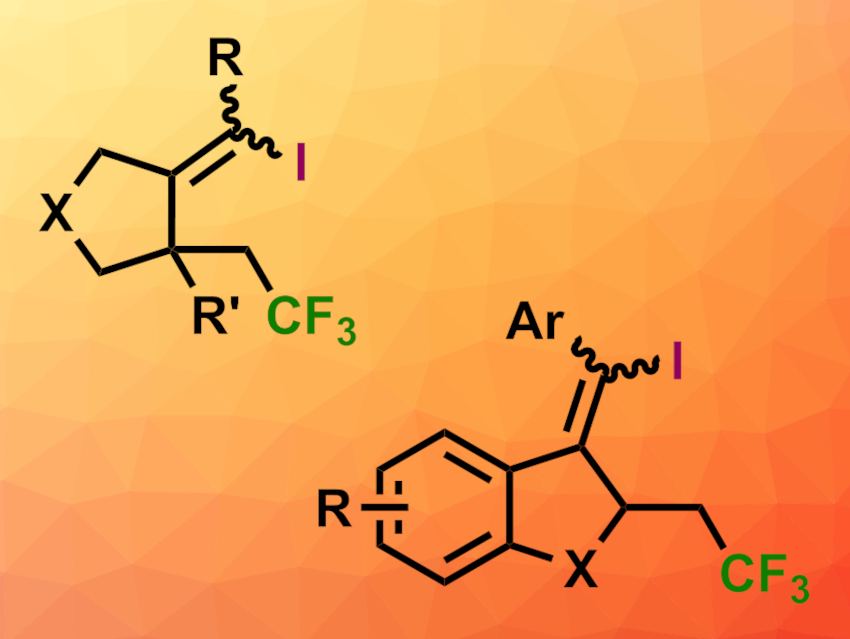
Jinhui Yang and Dianjun Li, Ningxia University, Yinchuan, China, and colleagues have found that triphenylphosphine and lithium iodide can promote a visible-light-induced radical cascade of trifluoromethylation/5-exo-dig cyclization/iodination of 1,6-enynes (general product structures pictured; top: X = NTs, O; bottom: X = C, CO). The well-known Togni’s reagent II (1-(trifluoromethyl)-1,2-benziodoxol-3(1H)-one) was used as a CF3 source, and lithium iodide functions as an iodine source.
Mechanistically, the researchers expect a radical mechanism since the addition of radical inhibitors such as TEMPO (2,2,6,6-tetramethylpiperidin-1-oxyl) leads to lower yields. The reaction shows good regioselectivity and functional group tolerance and proceeds under ambient conditions. The products are highly functionalized and could be useful for further transformations.
- Visible-Light-Enabled Ph3P/LiI-Promoted Tandem Radical Trifluoromethylation/Cyclization/Iodination of 1,6-Enynes with Togni’s Reagent,
Hui Liu, Xu Fan, Jinkai Hu, Tongtong Ma, Feng Wang, Jinhui Yang, Dianjun Li,
J. Org. Chem. 2022.
https://doi.org/10.1021/acs.joc.2c01453