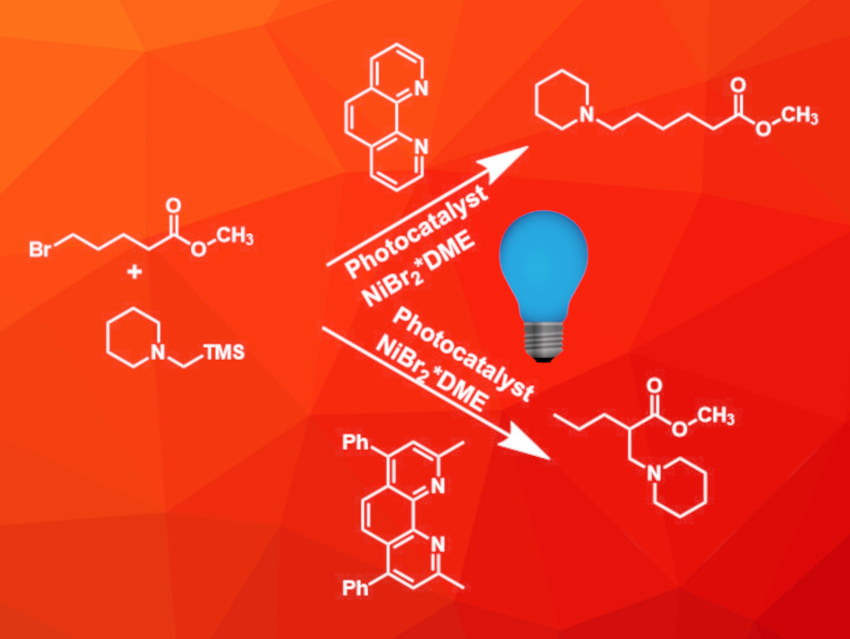
Tertiary amines with different alkyl substituents are important building blocks for natural products and pharmaceutically active compounds. However, the selective synthesis of these compounds can be quite challenging, especially if unactivated alkyl halides are used as starting materials.
Genping Huang, Tianjin University, China, Weiming Yuan, Huazhong University of Science and Technology (HUST), Wuhan, China, and colleagues have developed an aminoalkylation reaction between alkyl halides and silylamines using a combination of a nickel catalyst and a photoredox catalyst under blue LED light at room temperature (example pictured). The reaction was carried out in N-methylpyrrolidone using dual photoredox/nickel catalysis.
During a screening of different ligands, the team discovered that the ligand can be used to control the regioselectivity of the reaction. If simple 1,10-phenanthroline was used, an ipso-substituted product was obtained (pictured above, top row). Switching to a 2,9-dimethyl-4,7-diphenyl-substituted phenanthroline derivative, however, led to a remote-substituted product (pictured above, bottom row). Since both phenanthroline-based ligands are commercially available, this allows easy tuning of the reaction to obtain two different products from the same starting materials.
The ipso-coupling is compatible with primary and secondary alkyl halides with ester groups as well as other functional groups like cyano, olefin, and hydroxy groups. Amides showed lower reactivity than esters, and tertiary halides were not converted successfully. Benzyl bromide is not compatible with the reaction conditions, but benzyl chloride was used successfully. For the remote functionalization, tertiary alkyl halides can be used, and starting materials with amide groups are suitable, as well. Both reactions are compatible with a wide range of silylamines including complex cyclic compounds. Overall, the developed reaction provides access to various tertiary amines with different structures and functional groups.
- Nickel/Photoredox Dual-Catalyzed Regiodivergent Aminoalkylation of Unactivated Alkyl Halides,
Wenlong Wang, Xueyuan Yan, Fu Ye, Songlin Zheng, Genping Huang, Weiming Yuan,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c09705