Organoboron compounds are useful intermediates in organic synthesis. Multicomponent coupling reactions involving three or more building blocks can be a convenient way to build organoboron compounds in one step. One approach to this is the copper-catalyzed carboboration of alkynes. However, controlling the regioselectivity of these reactions can be challenging.
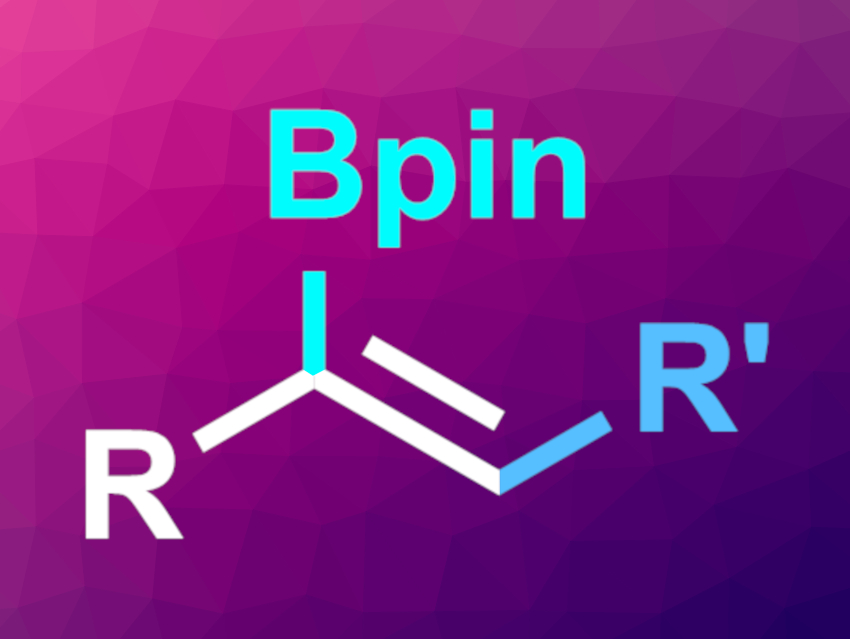
Guy Bertrand, Rodolphe Jazzar, University of California, San Diego, La Jolla, USA, Keary M. Engle, The Scripps Research Institute, La Jolla, CA, USA, and colleagues have developed a regioselective, copper-catalyzed three-component carboboration of terminal alkynes (general product structure pictured) using cyclic(alkyl)(amino)carbene (CAAC) ligands.
The team reacted a range of terminal alkynes with a variety of carbon electrophiles (including allyl alcohol derivatives and alkyl halides) and bis(pinacolato)diboron (B2pin2) to obtain the desired carboboration products. They used a (CAAC)CuCl complex with a five-membered, ethyl-functionalized cyclic (alkyl)(amino)carbene ligand (EtCAAC5) as the catalyst, LiOtBu as a base, and dimethylacetamide (DMA) as the solvent. The reactions were performed at room temperature.
The desired products were obtained in moderate to high yields. The reaction achieves high levels of α-selectivity for different carbon electrophiles. These trisubstituted alkenylboron compounds can be challenging to access otherwise.
- (CAAC)Copper Catalysis Enables Regioselective Three-Component Carboboration of Terminal Alkynes,
Yang Gao, Nana Kim, Skyler D. Mendoza, Sima Yazdani, Andre Faria Vieira, Mingyu Liu, Aaron Kendrick, Douglas B. Grotjahn, Guy Bertrand, Rodolphe Jazzar, Keary M. Engle,
ACS Catalysis 2022.
https://doi.org/10.1021/acscatal.2c00614