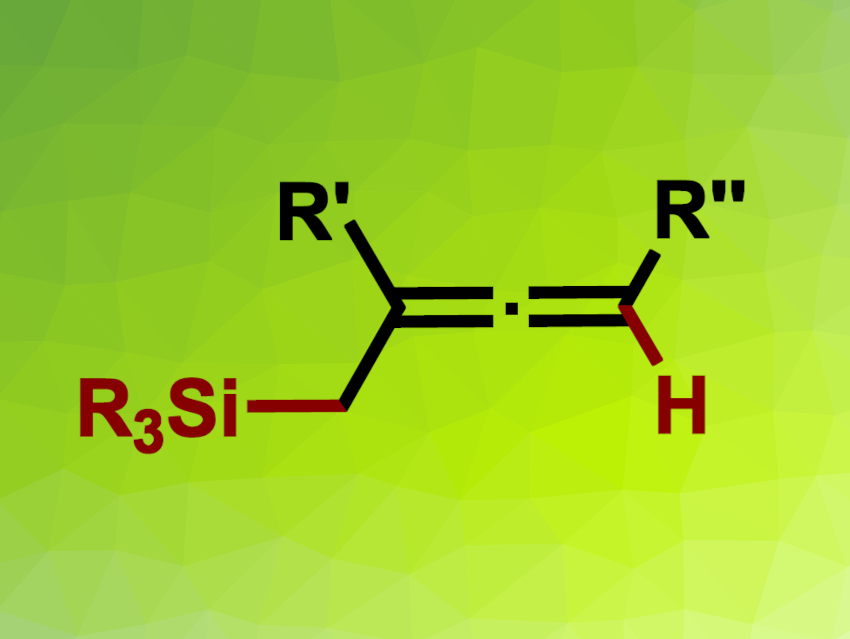
Allenes are useful intermediates in organic synthesis. Methods for their synthesis can suffer from drawbacks such as a need for harsh reaction conditions, prefunctionalized reactants, or unstable reagents. Thus, new approaches to the preparation of allenes are useful.
Guodong Zhang, Feng Chen, Yangzhou University, Jiangsu, China, Duo Zhang, Guangxi University of Science and Technology, China, and colleagues have developed a method for the regioselective hydrosilylation of 1,3-enynes that gives α-silylallenes (general product structure pictured). The approach is based on cooperative photoredox and nickel catalysis. The team reacted a range of 1,3-enynes with different silanecarboxylic acids using 2,4,5,6-tetra(9H-carbazol-9-yl)isophthalonitrile (4CzIPN) as a photocatalyst, Ni(DME)Br2 (DME = 1,2-dimethoxyethane) as the nickel catalyst, 4,4′-di-tert-butyl-2,2′-dipyridyl (dtbpy) as a ligand, K2HPO4 as a base, and methanol as the solvent. The reactions were performed under blue LED irradiation and an argon atmosphere at room temperature.
The desired α-silylallenes were obtained in mostly moderate to excellent yields. The reactions can also be used for deuterosilylations, giving α-silyldeuteroallenes when deuterated methanol is used as the solvent. The team proposes a reaction mechanism that involves the formation of a carboxyl radical from the silanecarboxylic acid via single electron transfer (SET), followed by decarboxylation. The resulting silyl radical undergoes a radical addition to the 1,3-enyne, giving a propargyl radical, which isomerizes to an allenyl radical and then forms an allenyl nickel complex. A protonation and another SET step then give the product and regenerate the catalysts.
- Regioselective Hydro(deutero)silylation of 1,3-Enynes Enabled by Photoredox/Nickel Dual Catalysis,
Guodong Zhang, Wei Tan, Duo Zhang, Kaiping Wang, Pan Gao, Shuli Wang, Shuang-Liang Liu, Feng Chen,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.3c04064