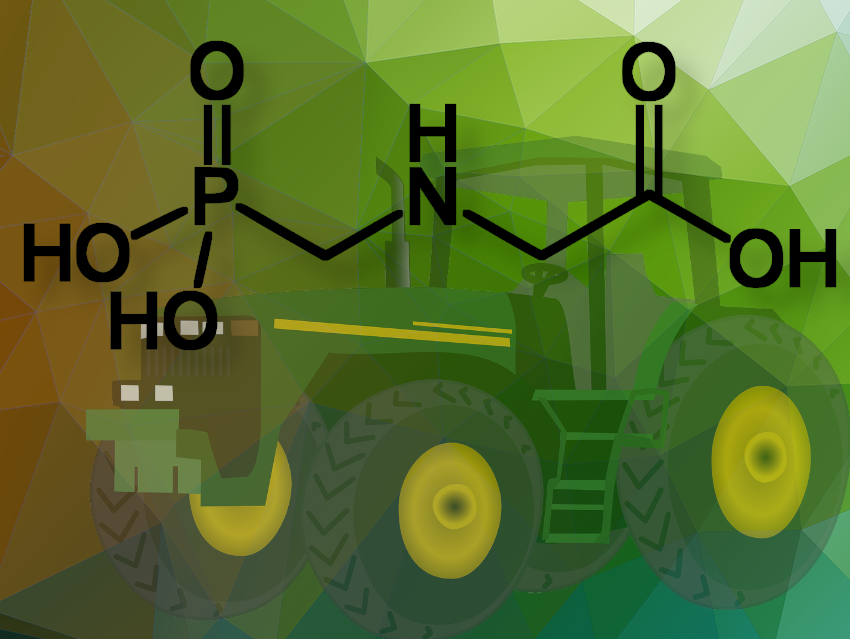
The European Member States have failed to reach the required majority to renew or reject the approval of glyphosate during an Appeal Committee vote. Glyphosate (N-(phosphonomethyl)glycine) is one of the most commonly used active substances in pesticides to prevent unwanted plant growth around crops or to kill plants or parts of plants. It has raised concerns due to its potential environmental and health impacts.
EU legislation requires the Commission to make a decision before the current approval expires on December 15, 2023. Based on comprehensive safety assessments by the European Food Safety Authority (EFSA) and the European Chemicals Agency (ECHA) as well as the EU Member States, the Commission will now renew glyphosate’s approval for ten years. This renewal comes with new conditions, such as prohibiting pre-harvest use and implementing measures to protect non-target organisms.
Member States maintain responsibility for authorizing glyphosate-containing plant protection products (PPPs) nationally and regionally, with the flexibility to impose restrictions based on risk assessments, particularly to safeguard biodiversity.
The scientific evaluation took into account a wide range of information, including mandatory EU regulatory studies and an extensive amount of published scientific literature. Reviewing over 16,000 studies, the applicants considered about 2,000 to be relevant, which were further narrowed down to 780 publications after comprehensive assessment. Additionally, 300 studies were brought to the committee’s attention during a public consultation.
In 2022, the European Chemicals Agency (ECHA) carried out a hazard assessment of glyphosate and concluded that it did not meet the scientific criteria to be classified as a carcinogenic, mutagenic or reprotoxic substance but agrees to keep the current classification of glyphosate as causing serious eye damage and being toxic to aquatic life with long-lasting effects. EFSA’s conclusion and supporting documents are publicly available on their website.
- No qualified majority reached by Member States to renew or reject the approval of glyphosate, Statement 3/5792 of European Commission, Brussels, Belgium, November 16, 2023. (accessed Nobemver 17, 2023)
- Peer review of the pesticide risk assessment of the active substance glyphosate,
European Food Safety Authority (EFSA), Fernando Álvarez, Maria Arena, Domenica Auteri, Marco Binaglia, Anna Federica Castoldi, Arianna Chiusolo, Federica Crivellente, Mark Egsmose, Gabriella Fait, Franco Ferilli, Varvara Gouliarmou, Laia Herrero Nogareda, Alessio Ippolito, Frederique Istace, Samira Jarrah, Dimitra Kardassi, Aude Kienzler, Anna Lanzoni, Roberto Lava, Alberto Linguadoca, Christopher Lythgo, Iris Mangas, Laura Padovani, Martina Panzarea, Juan Manuel Parra Morte, Simone Rizzuto, Anamarija Romac, Agnès Rortais, Rositsa Serafimova, Rachel Sharp, Csaba Szentes, Andrea Terron, Anne Theobald, Manuela Tiramani, Giorgia Vianello, Laura Villamar-Bouza,
J. EFSA 2023.
https://doi.org/10.2903/j.efsa.2023.8164