Cleaving C–C bonds in arenes via the insertion of one or more atoms is generally challenging due to the stability of aromatic compounds. However, such reactions could be useful to dearomatize and upconvert arenes to synthetically valuable species. Insertions of transition metal atoms are known, while insertion reactions with main group metals or metalloid elements can be more difficult. Insertions of a boron atom into aromatic C–C bonds, in particular, are rare, despite the possible utility of the products.
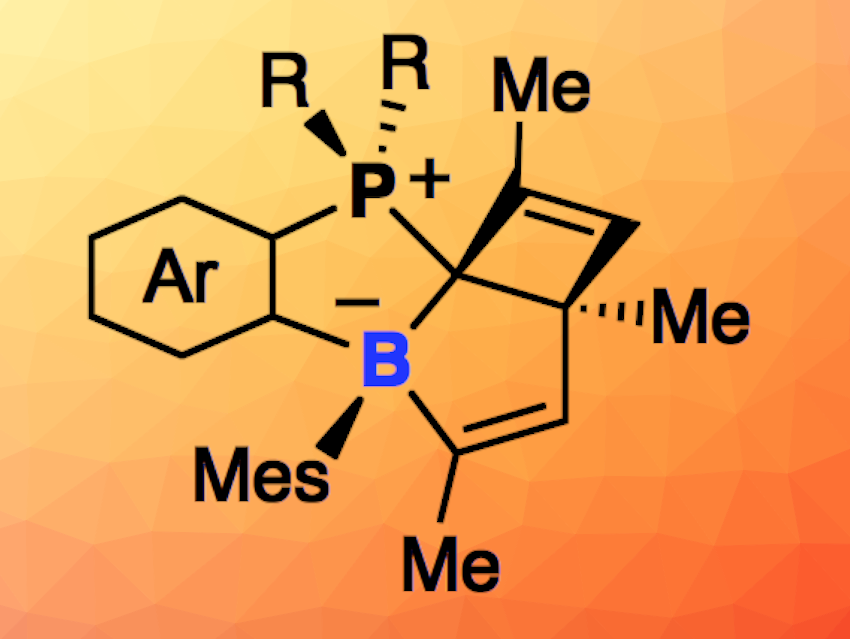
Jun Takaya, Tokyo Institute of Technology, Japan, and colleagues have observed an unprecedented formation of borabicyclo[3.2.0]heptadiene derivatives via a reversible boron insertion into aromatic C–C bonds in a photo-promoted skeletal rearrangement reaction of ambiphilic phosphine-boranes (pictured below). The team irradiated a phosphine-borane with a 1,2-naphthylene linker between phosphorus and boron in [D6]benzene at 6 °C and obtained a zwitterionic phosphonium-borate compound with a cis-borabicyclo[3.2.0]hepta-3,6-diene skeleton.
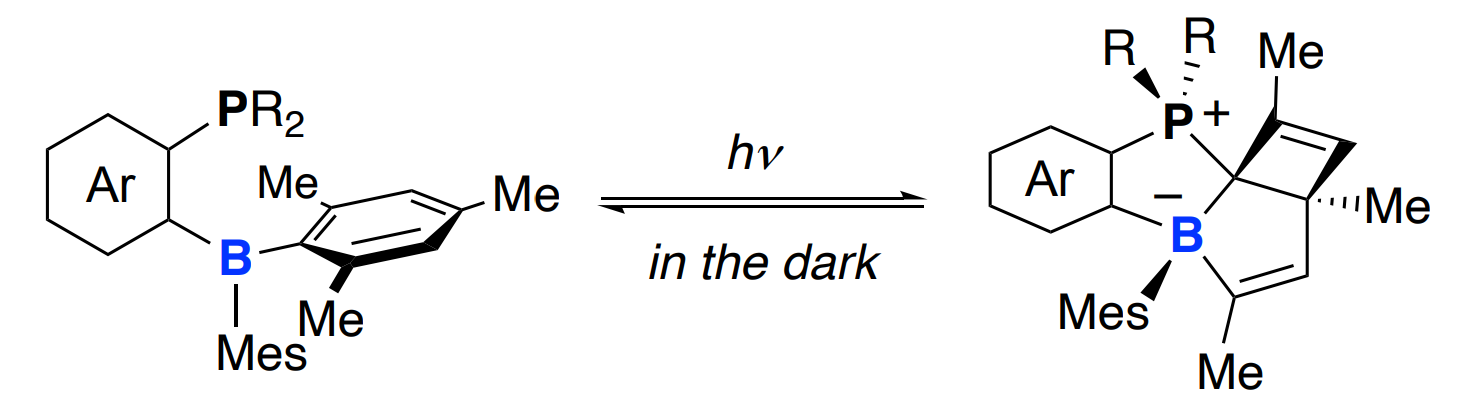
The product was converted back to the corresponding ambiphilic phosphine-borane quantitatively at room temperature in the dark after 35 h. Thus, the researchers realized a reversible boron insertion into an aromatic C–C bond that is controlled by light and heat. According to the team, the reaction mechanism features sequential electrocyclization reactions involving the E/Z-isomerization of an alkene unit via a highly strained trans-borepin intermediate. The boron-containing products could serve as useful synthetic building blocks.
- Reversible Boron‐insertion into Aromatic C–C Bonds,
Kaito Kuroki, Tatsuyoshi Ito, Jun Takaya,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202312980