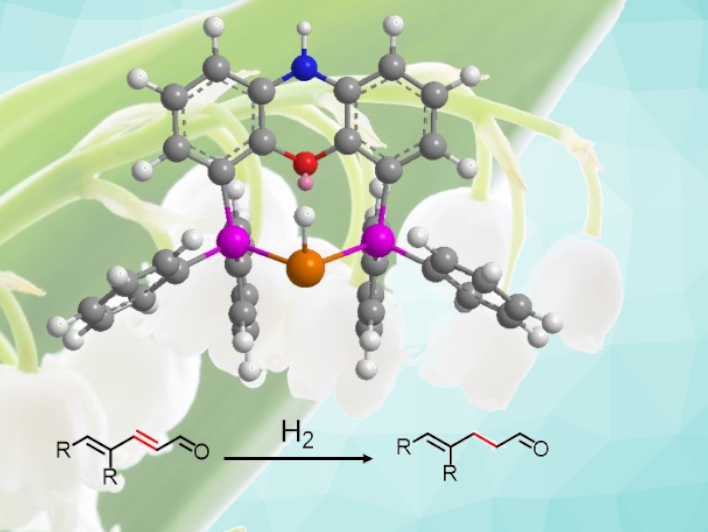
Aldehydes are often used in the perfume industry. Muguet aldehydes, from the French word for lily of the valley, for example, have floral notes and are highly appreciated in the industry. There are γ,δ-unsaturated aldehydes in this family that are usually prepared by a Claisen rearrangement. However, the high temperatures or acidic conditions required by this approach can be problematic with respect to the stability of the formed aldehyde. Therefore, greener and milder synthetic approaches would be useful.
Lionel Saudan, Firmenich SA, Geneva, Switzerland, and colleagues have developed a rhodium complex that can selectively catalyze the hydrogenation of conjugated dienals into γ,δ-unsaturated aldehydes (pictured). The rhodium complex was formed in situ from commercially available materials, i.e., [Rh(COD)Cl]2 (COD = 1,5-cyclooctadiene), a diphosphine ligand, and potassium benzoate. The team used large-bite-angle diphosphines because only diphosphines with a bite angle over ca. 100° resulted in an active and regioselective catalyst. Xantphos with a bite angle of 111° and nixantphos, which has a bite angle of 114°, were found to be suitable.
Using the developed catalyst system, a variety of dienals were α,β-hydrogenated under 10–50 bar H2, using, e.g., ethanol as the solvent. The reaction proceeded under mild conditions and the desired γ,δ-unsaturated aldehydes were obtained in high yields.
- Highly Selective Rhodium Catalyzed 1,4‐Hydrogenation of Conjugated Dienals,
Christophe M Saudan, Aurelien Berrocosa, Julie Quintaine, Stéphanie Spoehrle, Laurent Maggi, Hervé Mosimann, Lionel Saudan,
ChemCatChem 2022.
https://doi.org/10.1002/cctc.202200671