The selective transformation of lignin to value-added compounds in high yields can be very challenging due to its structural complexity and the vast number of possible reaction pathways. The yield of isolated phenolic acids (PAs), key base chemicals for biobased polyesters, directly from lignin is typically below 5 wt%. Methods for the depolymerization of lignin to PAs in high yields under mild reaction conditions are, thus, interesting research targets.
Dequan Xiao, University of New Haven, West Haven, CT, USA, Zhaohui Tong, Georgia Institute of Technology, Atlanta, USA, and colleagues have developed an effective route to selectively convert lignin extracted from sweet sorghum and poplar into isolated PAs in high yields. The team used a low-cost graphene oxide-urea hydrogen peroxide (GO-UHP) catalyst system under mild conditions. First, an acetylation blocks the lignin’s phenolic OH groups, which allows the non-metal catalyst GO to selectively depolymerize lignin to aromatic aldehydes under mild conditions (<120 °C, ambient pressure). Then, the aromatic aldehydes in the depolymerized lignin can be upgraded by a low-cost oxidizer (UHP) to produce aromatic carboxylates, followed by a simple base/acid extraction process to isolate the desired PAs from other low molecular weight aromatics.
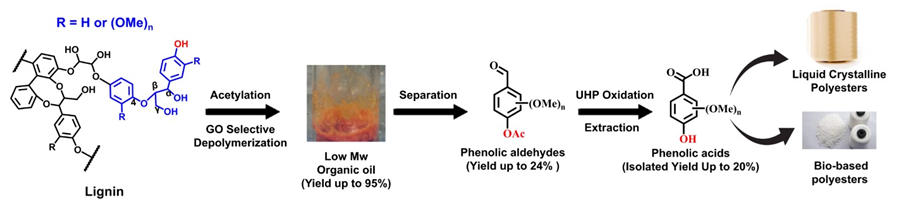
The lignin depolymerization achieved a lignin conversion yield of to 95 %, and the yield of isolated PAs was as high as 20 wt%. The residual organic oil has potential for utilization in aviation fuel production. Overall, the work provides a new way to selectively cleave lignin side chains to give isolated aromatic monomers.
- Selective Depolymerization of Lignin Towards Isolated Phenolic Acids Under Mild Conditions,
Wenbo Peng, Hanxi Bao, Yigui Wang, Elizabeth Cote, William J. Sagues, Halena Hagelin-Weaver, Ji Gao, Dequan Xiao, Zhaohui Tong,
ChemSusChem 2023.
https://doi.org/10.1002/cssc.202300750




