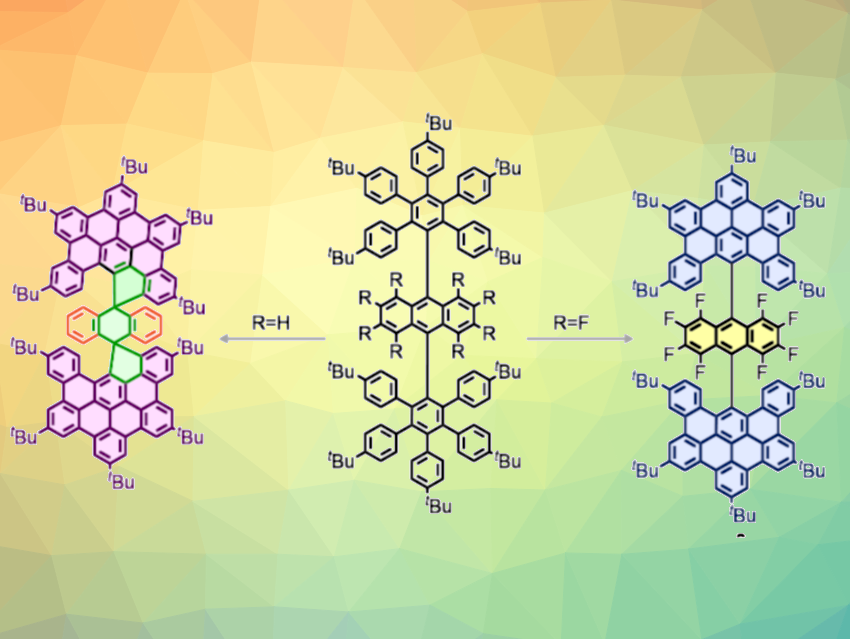
Synthetic molecular nanographenes are commonly obtained by the oxidation of polyphenylene precursors with oxidants under strongly acidic conditions. This key graphitization step, known as the Scholl oxidation, is an intramolecular reaction that leads to the formation of multiple aryl–aryl bonds. The mechanism depends greatly on the electronic effects of the substrate and the nature of the reagents, which makes the Scholl reaction unpredictable in some cases.
Nazario Martín, Israel Fernández, Complutense University of Madrid, Spain, and colleagues have successfully controlled the direction of the Scholl reaction toward spiro or helical nanographenes using electronic effects. The team synthesized rare spironanographenes (example pictured above on the left) from anthracene-based polyphenylenes (pictured above in the center, R = H) in the presence of either FeCl3 (Lewis acid and oxidant) or dichlorodicyano-p-benzoquinone (DDQ) as the oxidant together with triflic acid as a Brønsted acid. Theoretical calculations showed that an intermediate containing a highly stabilized trityl cation forms to give two spirocycles, followed by graphitization.
In contrast, when eight fluorine atoms are attached to the anthracene core of the polyphenylene, a helical nanographene is generated (pictured above on the right). With this electron-deficient anthracene substrate, the dehydrogenation process is controlled by the electronic properties of the fluorinated anthracene core, and the graphitization of the polyphenylene takes place selectively without the formation of spirocycles. The helical nanographene has interesting photophysical properties that may be useful in optoelectronic applications, according to the researchers.
- Electronic Control of the Scholl Reaction: Selective Synthesis of Spiro vs Helical Nanographenes,
Patricia Izquierdo-García, Jesús Manuel Fernández-García, Josefina Perles, Israel Fernández, Nazario Martín,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202215655