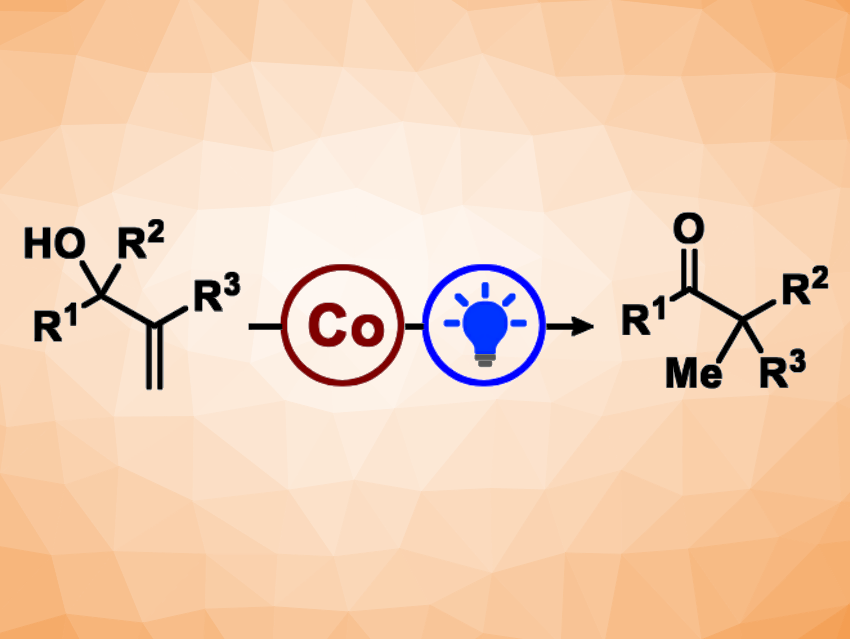
The semipinacol rearrangement is a rearrangement reaction that usually involves carbocations. Cationic semipinacol rearrangements are traditionally performed using Brønsted or Lewis acids. Erick Moran Carreira, Swiss Federal Institute of Technology (ETH) Zurich, Switzerland, and colleagues have developed a photo- and cobalt-catalyzed semipinacol rearrangement of unactivated allylic alcohols (general reaction pictured).
The team used a benzothiazinoquinoxaline as an organic photocatalyst, a cobalt salen complex as a transition-metal catalyst, lutidinium triflate as a proton source, and CH2Cl2 as the solvent. They converted a variety of allylic alcohols to the corresponding α,α-disubstituted ketones under blue LED light. The desired products were obtained in mostly good to high yields, and the reaction tolerates both aliphatic and aromatic migrating groups.
According to the team, the reactivity of their catalytic system is complementary to that observed with Brønsted acids. The reaction proceeds under mild conditions and is compatible with ethers, esters, halides, nitriles, carbamates, and substituted arenes. No stoichiometric reductant or oxidant is necessary. The approach can be used for late-stage diversifications of molecules with complex scaffolds. The researchers propose that the combination of organophotoredox and transition-metal catalysis could continue to expand the horizon of catalytic chemical methods.
- Cobalt‐Catalyzed Photo‐Semipinacol Rearrangement of Unactivated Allylic Alcohols,
Erick Moran Carreira, Henry Lindner,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202407827