Explosives that use easily accessible peroxides, nitrates, or chlorates can be used to build improvised explosive devices (IEDs), e.g., by terrorists and other criminals. Detecting these substances is, thus, important for safety applications. In places such as airports, the infrastructure can allow for the use of instrumental analytical methods by trained personnel. However, portable, easy-to-use methods could be helpful in other settings, such as in the field for first responders or for mass testing.
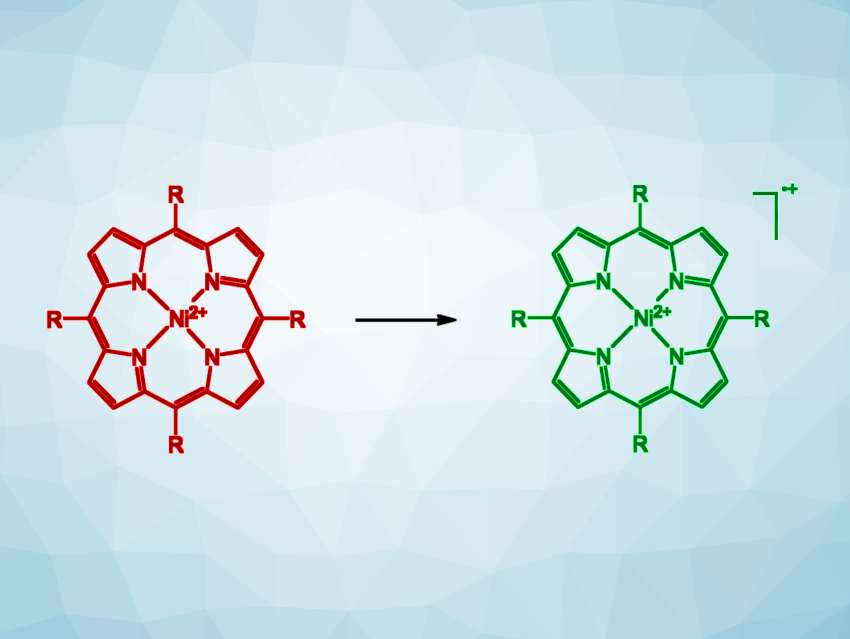
Georg Gescheidt, Graz University of Technology, Austria, Rainer Herges, University of Kiel, Germany, and colleagues have developed a simple method for the detection of traces of “homemade” explosives via a color change, similar to a pH test strip. The team used an electron-rich nickel porphyrin (simplified structure pictured in red, R = 3,4,5-trimethoxyphenyl) as a redox-responsive indicator.
This complex can be oxidized to the corresponding radical cation, leading to a color change from red to green, by peroxide-based explosives, such as triacetone triperoxide (TATP), in the presence of acids such as trifluoroacetic acid (TFA) or perfluoropentanoic acid. The same nickel porphyrin can also be used for the detection of inorganic nitrates or chlorate-based explosives, which can oxidize the complex further to a dication, leading to a color change to brown.
The researchers created test strips containing the nickel porphyrin complex. They initially show a red color. The strips can be activated with perfluoropentanoic acid and then used to test for the targeted explosives. For explosives with a relatively high vapor pressure such as TATP, the strip can be used for safe, contactless detection via the gas phase by holding it close to the sample. Overall, the team’s approach allows the sensitive, simple, robust, and portable detection of homemade explosives made from peroxides, nitrates, or chlorates.
- Highly Sensitive, Easy-to-Use, One-Step Detection of Peroxide-, Nitrate- and Chlorate-Based Explosives with Electron-Rich Ni Porphyrins,
Mike Brockmann, Gabriel Glotz, Jan-Simon von Glasenapp, Lara Unterriker, Dmytro Neshchadin, Georg Gescheidt, Rainer Herges,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c14118