C–H functionalizations in allylic (or propargylic) positions give useful intermediates for further transformations in organic synthesis. In substrates with two different allylic protons, site selectivity can be a problem in such reactions. The effects of electron-withdrawing substituents can be used to increase the difference between the two C–H bonds and improve the site selectivity. However, the use of electron-donating groups in this context had not been reported so far.
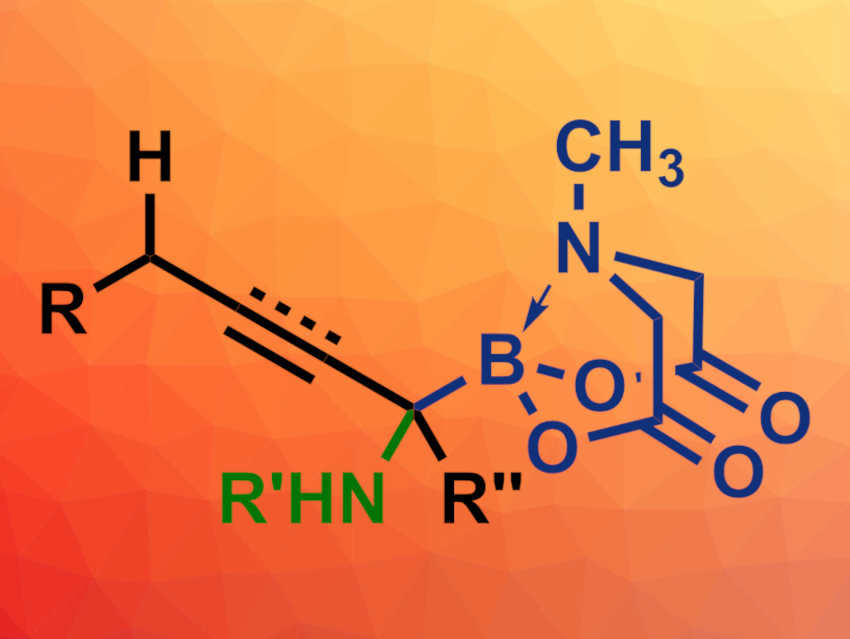
Honggen Wang, Sun Yat-Sen University, Guangzhou, China, and colleagues have developed a boryl-directed intermolecular C–H amination of allyl N-methyliminodiacetyl boronates or propargylic N-methyliminodiacetyl boronates (B(MIDA)s) to give α-amino boronates with high site selectivity (general product structure pictured). In this approach, an electron-donating B(MIDA) group directs a selenium-catalyzed amination.
The team reacted a variety of primary and secondary allyl MIDA boronates with 2,2,2-trichloroethoxysulfonamide (TcesNH2) as an amino source, using 1,3-dimethyl-1,3-dihydro-2H-im
The desired α-amino boronates were obtained in generally good to excellent yields and with high selectivities. The highly functionalized products are useful substrates for further reactions.
- Boryl-Dictated Site-Selective Intermolecular Allylic and Propargylic C–H Amination,
Yuan Liu, Zhi-Hao Chen, Yin Li, Jiasheng Qian, Qingjiang Li, Honggen Wang,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c06117