B. Terzi and W. Teich (LMU Munich) report the first light-sensitive m⁷Gp₄G RNA cap analogs that enable precise, light-controlled regulation of RNA translation
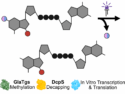
Light-Activated mRNA Cap Analogs Allow Timed Control of mRNA Translation

ChemBioChem Readers’ Choice 2025
Readers' Choice collection at ChemBioChem—enjoy reading the top articles from 2023 and 2024 selected based on citations, downloads, and social media engagement
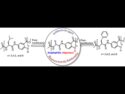
Site-Modified Foldamers Target Pathogens Fast
Precision-modified foldamers show promise in targeting drug-resistant bacteria, offering a new path for therapeutic development.
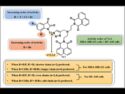
Smart Hybrids Fight Breast and Prostate Cancer
Potent triazole-tethered hybrids show strong cancer-killing action against TNBC and prostate lines, offering hope for smarter, targeted treatment options.

Chemistry – A European Journal Readers’ Choice 2025
Collection that showcases the most popular articles published in the journal in 2023 and 2024 in terms of citations, downloads, social media activity
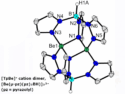
Sterics, Electronics, and Reactivity in Beryllium Phosphine Complexes
Magnus Buchner, Marburg, and his coauthors have synthesized and characterized monocationic tris(pyrazolyl)borate beryllium phosphine complexes

Decoding Protein Disorder: Peptides, IDPs, Therapeutic Insights
Short peptides offer new clues to protein behaviour, unlocking how disordered proteins function and paving the way for disease research and drug design.
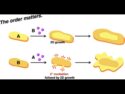
Sequence Matters: Building Smart 2D Block Copolymers
Discover how sequencing monomers leads to unique 2D block copolymers, offering new strategies for designing functional materials for electronics and sensing.
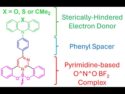
Metal-Free Boron Emitters Powering the Future of OLEDs
New boron compounds show strong thermally activated delayed fluorescence (TADF), offering sustainable, efficient path to next-gen OLEDs without rare metals
Triazole Power Play: New Hybrids Target Diabetes with Precision
New adamantane-triazole molecules show strong α-glucosidase inhibition, outperforming acarbose and revealing promising antidiabetic potential