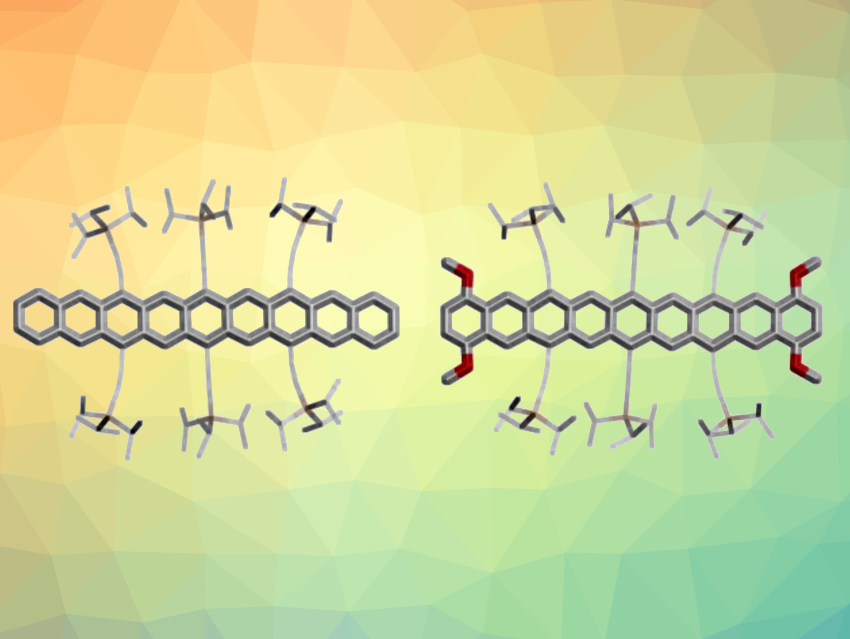
Acenes are polycyclic aromatic hydrocarbons composed of linearly annulated benzene rings. They are used, e.g., as semiconductors in organic field-effect transistors (OFETs), and larger acenes tend to provide better properties. However, the synthesis of higher acenes (larger than heptacene) is challenging, because they have a tendency to undergo decomposition reactions.
Uwe H. F. Bunz, University of Heidelberg, Germany, and colleagues have developed a synthetic route that provides access to four soluble and persistent nonacenes (example structures pictured). Each product has six strategically placed triisopropylsilyl (TIPS)-ethynyl groups, and additionally, two are halogenated and one is methoxylated. First, anthraquinones were reacted with TIPS-ethynylated diepoxyanthracene and tetrazine. A deoxygenation, followed by a fourfold ethynylation with lithiated TIPS-acetylene gave tetraol intermediates. Finally, a reductive aromatization gave the desired nonacenes.
The sterically shielding TIPS substituents and the halogenation or methoxylation of the nonacene backbone make the compounds persistent in solution. Their stability allows for easy handling and characterization. The researchers also processed one derivative into organic thin-film transistors (OFETs)—the first example of a nonacene-based OFET. The compounds described are the longest-lived nonacenes to date. The stability and solubility of the materials can be important for fundamental studies and for allowing semiconductor applications of higher acenes.
- Hexakis‐TIPS‐Alkynylated Nonacenes: Persistent and Processible,
Nico Zeitter, Nikolai Hippchen, Patrick Jäger, Anna Weidlich, Philipp Ludwig, Frank Rominger, Andreas Dreuw, Jan Freudenberg, Uwe Heiko Bunz,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202302323