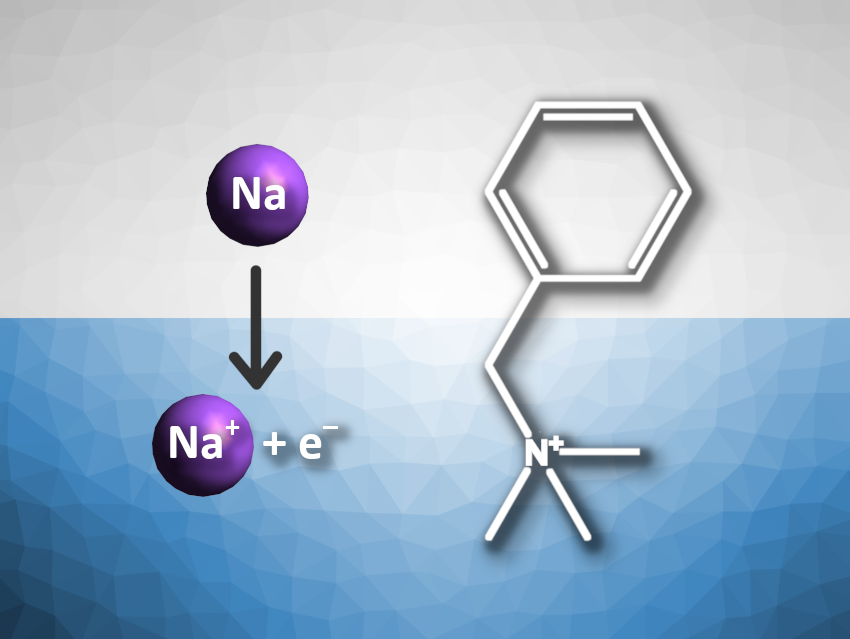
Solvated electrons are very strong reductants. Many different reactions of solvated electrons in bulk water have been studied. At or near the water–vapor interface, they could react differently due to differences in solvation. Solvated electrons can be created at the surface of water, e.g., by exposing a small jet of water in a vacuum to gas-phase sodium atoms (pictured above on the left). To make sure that the electrons’ reaction partners are also located at the interface, looking at reactions with surfactants is a useful approach.
Gilbert M. Nathanson, University of Wisconsin-Madison, USA, and colleagues have investigated the reaction of solvated electrons with the surfactant benzyltrimethylammonium (BTMA+, pictured above on the right). BTMA+ can react with solvated electrons to give a benzyl radical and trimethylamine. The team used a microjet scattering apparatus in a vacuum with an aqueous solution of LiBr and BTMACl. A beam of sodium atoms from an oven was directed at the liquid jet. The experiments were performed at 235 K to minimize water evaporation and at pH 2 to prevent the hydrolysis of BTMA+ by OH–. Evaporating benzyl radicals and trimethylamine molecules were detected using mass spectrometry.
The team found that the products formed at or near the interface can escape before they react further in solution. The formed trimethylamine can reach the gas phase before it is protonated. Similarly, benzyl radicals escape before they can combine with hydrogen atoms or other benzyl radicals to form toluene or bibenzyl, respectively. The team calculated that the electrons react within 20 Å of the surface on average. Overall, the work could be useful for developing methods to explore radical chemistry near the interface via the evaporation of reaction intermediates into the gas phase.
- Creation and Reaction of Solvated Electrons at and near the Surface of Water,
Xiao-Fei Gao, David J. Hood, Xianyuan Zhao, Gilbert M. Nathanson,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c03370