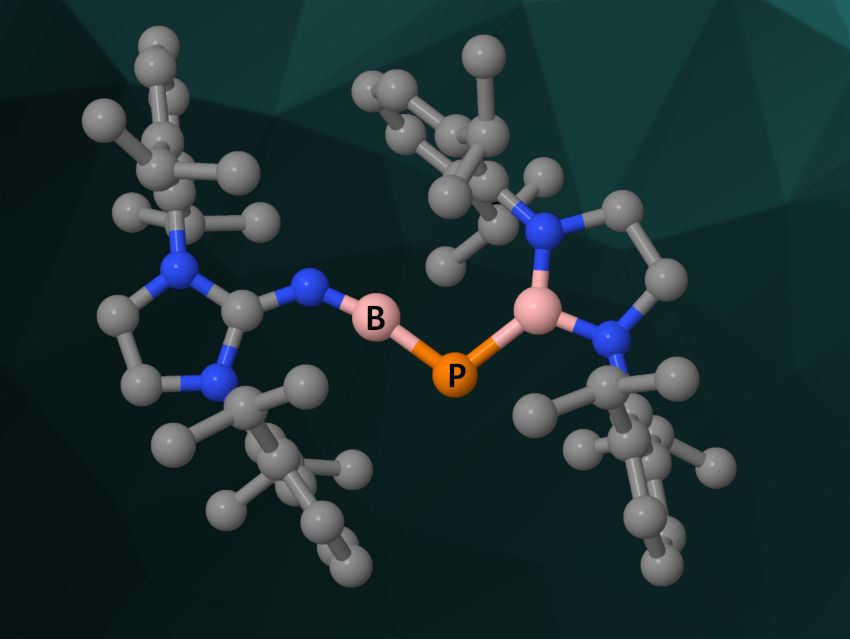
Analogues of organic compounds with heavier main-group elements or isoelectronic groups are often interesting research targets. Multiple bonds, in particular, easily form between C atoms but can be challenging to stabilize for heavier elements. Free phosphaborenes (R–P═B–R), for example, are PB analogues of alkynes. They are difficult to isolate because the P–B double bond is labile and phosphaborenes tend to oligomerize. So far, free phosphaborenes have only been detected in the gas phase and not observed as isolable species in the condensed phase at room temperature.
Liu Leo Liu, Southern University of Science and Technology, Shenzhen, China, and colleagues have synthesized and isolated a crystalline free phosphaborene (pictured) at room temperature. The team combined one π-donating and one π-accepting substituent with bulky aromatic groups at the outer ends to stabilize the target product. The π-donating substituent is situated at the boron atom and reduces its Lewis acidity, while the π-accepting substituent is connected to the phosphorus atom and reduces its Lewis basicity. These effects work together to inhibit oligomerization. The bulky aromatic substituents provide additional kinetic stabilization.
The researchers started from a 2,6-diisopropylphenyl (Dipp)-substituted bromodiazaborolidine, which was converted to a Dipp-substituted phosphane via a salt metathesis reaction with sodium phosphide. This intermediate was successively reacted with benzyl potassium, trimethylsilyl chloride (TMSCl), and tBuLi to obtain a lithiated building block. This building block was then reacted with a Dipp-substituted boron dibromide precursor to create the skeleton of the target product. In a final step, TMSBr was eliminated to give the free phosphaborene. The product can be stored in the solid state under a nitrogen atmosphere for weeks, but it is extremely sensitive to moisture.
- A Free Phosphaborene Stable at Room Temperature,
Jiancheng Li, Zhihao Lu, Liu Leo Liu,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c11878