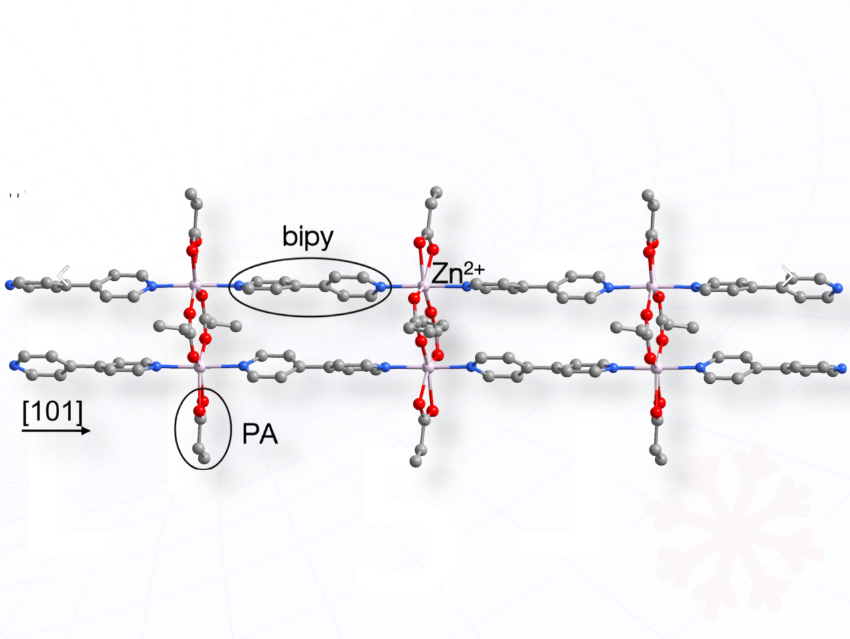
Ming-Sheng Wang, Guo-Cong Guo, and colleagues, Chinese Academy of Sciences, Fuzhou, Fujian, P. R. China, have made a 1-D zinc coordination polymer ([Zn₂(PA)₄(bipy)₂]ₙ·nH₂O) by dissolving Zn₂(OH)₂CO₃, propionic acid (PA), and bipyridine (bipy) in a water–methanol mixture, then heating the solution at 100 °C for 30 hours in a sealed vessel. This solvothermal reaction produced colorless crystals of the polymer, which could then generate monovalent Zn⁺ species upon light irradiation.
The team found that light triggers electron transfer within the polymer, producing air-stable Zn⁺ that persists for at least a week, while the crystalline framework largely remains intact. The polymer changes color from colorless to light brown under light, signaling the creation of Zn⁺ and radical bipyridine.
According to the researchers, this provides a simple, room-temperature method to access Zn⁺ species, which could be useful for catalysis and studying unusual metal–electron interactions.
- Light-Driven Generation of Air-stable Zn(I) Under Ambient Conditions
Hong-Zhi Xiao, Mian-He Xu, Li-Zhen Cai, Ming-Sheng Wang, Guo-Cong Guo
Chem. Eur. J. 2025
https://doi.org/10.1002/chem.202502955