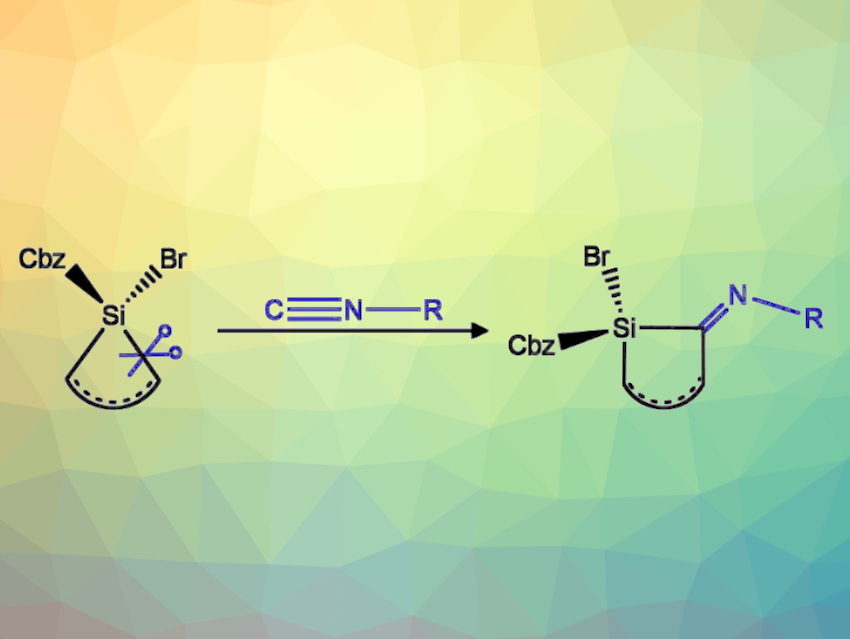
Cyclic organosilicon compounds are widely used, e.g., in pharmaceuticals, agrochemicals, dyes, or polymers. Small, ring-strained silicon-containing heterocycles are also interesting research targets due to their reactivity. They can, for example, undergo ring expansion reactions, often catalyzed by transition metals.
Maximilian P. Müller and Alexander Hinz, Karlsruhe Institute of Technology (KIT), Germany have synthesized small silacycles that can be treated with isonitriles to induce non-catalyzed ring expansion reactions (general reaction pictured above). The team reacted the bromosilylene [(dtbpCbz)SiBr] (dtbpCbz = 1,8-bis(3,5-ditertbutylphenyl)-3,6-ditertbutylcarbazol) with ethylene, acetylene, or tert-butyl phosphaacetylene to obtain saturated or unsaturated three-membered silacycles—siliranes or (phospha)silirenes, respectively. The precursor [(dtbpCbz)SiBr] was also treated with 1,3-dibromopropane and then reduced to give a siletane (a four-membered silacycle) as well as with dimethyl butadiene to give a 3-silolene (an unsaturated five-membered silacycle).
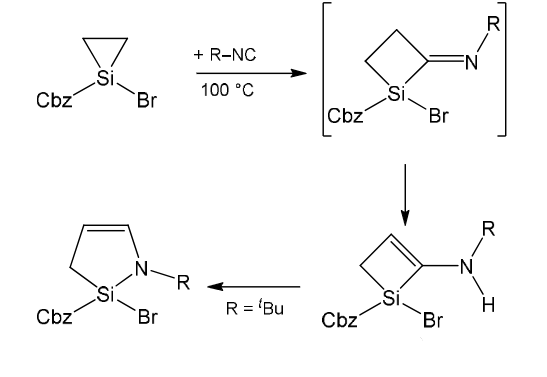
The synthesized silacycles were then subjected to reactions with the isonitriles CNDmp (Dmp = 2,6-dimethyl-phenyl) and CNtBu. Ring expansion reactions of three-membered silacycles were observed, leading to four-membered heterocycles. The ring-expansion products stemming from the saturated silirane were found to undergo further isomerisation reactions, first by enamine formation and then by another ring expansion (pictured above). Four- and five-membered silacycles could not be expanded under these conditions.
- Strain‐driven, non‐catalysed ring expansion of silicon heterocycles,
Maximilian P. Müller, Alexander Hinz,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202302311