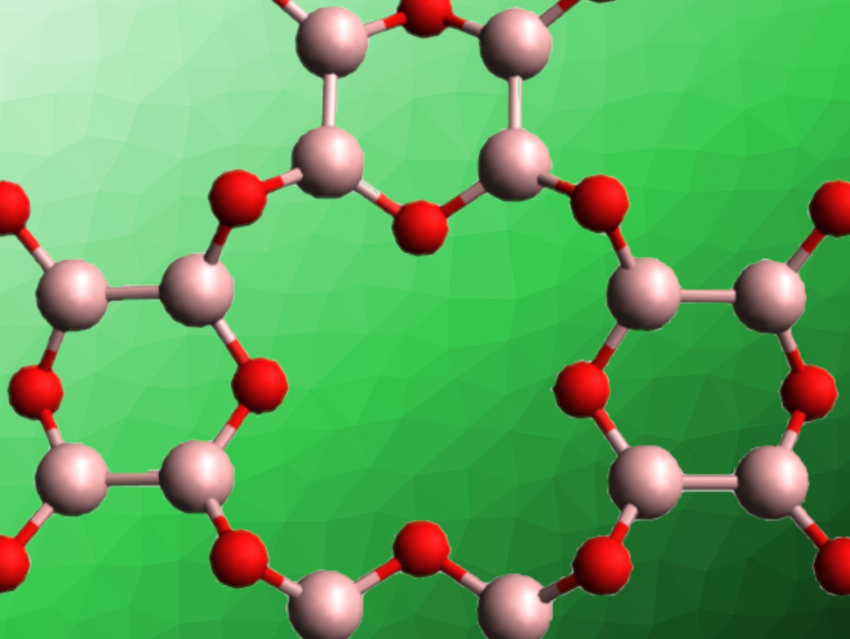
Boron monoxide (BO) can be formed via the condensation of tetrahydroxydiboron at elevated temperatures. However, the exact structure of the resulting material has not determined. Different molecular structures and 1D, 2D, or 3D polymeric structures have been suggested. Proposed layered 2D structures are composed of B4O2 or B3O3 units. The material’s reactivity indicates that B–B bonds are retained in boron monoxide.
Frédéric A. Perras, Wenyu Huang, Ames National Laboratory and Iowa State University, Ames, USA, and colleagues have used advanced 11B NMR experiments as well as powder diffraction data to investigate the structure of boron monoxide. The team first synthesized boron monoxide by exposing tetrahydroxydiboron to elevated temperatures of 200, 300, 400, or 700 °C. The resulting samples were investigated using solid-state NMR spectroscopy methods such as magic angle spinning (MAS), multiple-quantum (MQ)MAS, dipolar heteronuclear multiple-quantum coherence (D-HMQC), double-quantum-filtered (DQF) J-resolved NMR, symmetry-based resonance-echo double-resonance (S-REDOR), DQ/SQ (double quantum/single quantum) correlation, and triple-quantum single-quantum (TQ/SQ) correlation.
The team found that there were two resonances in 11B MAS solid-state NMR spectra, one of which they assigned to BO. The other appeared at synthesis temperatures of 300 °C and above. It was assigned to B2O3, which forms a separate phase from BO.
NMR spectra of the BO sample prepared at 200 °C, in combination with density functional theory (DFT) calculations, showed that the BO structure is probably two-dimensional, with planar, locally D2h-symmetrical O2B–BO2 units. This allowed the team to narrow down the structure to 2D sheets composed of either B3O3 or B4O2 rings. The TQ/SQ correlation spectra then agreed better with simulated spectra for a B4O2-based structure (pictured). Using X-ray powder diffraction, the researchers found evidence that these layers formed from connected B4O2 rings stack in a random pattern.
- The Structure of Boron Monoxide,
Frédéric A. Perras, Henry Thomas, Patrick Heintz, Ranjan Behera, Jiaqi Yu, Gayatri Viswanathan, Dapeng Jing, Scott A. Southern, Kirill Kovnir, Levi Stanley, Wenyu Huang,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c02070