The production of polymers using elemental sulfur can provide new materials and turn S8 into useful products. One approach for this is inverse vulcanization, in which molten elemental sulfur is used as a monomer and is co-polymerized with unsaturated organic monomers to prepare polymers with a high sulfur content. However, this method has a limited substrate scope due to the poor miscibility of some organic monomers in molten sulfur and the high temperatures required.
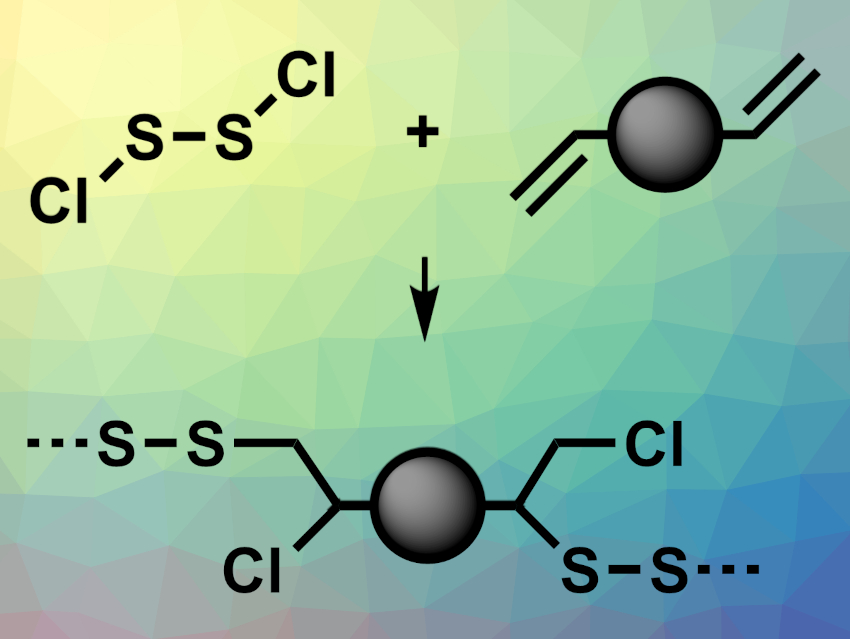
Robert A. Norwood, Jon T. Njardarson, Jeffrey Pyun, University of Arizona, Tucson, USA, and colleagues have developed a new process, called sulfenyl chloride inverse vulcanization (example reaction pictured), which uses S2Cl2 as an alternative, highly reactive, inexpensive sulfur-containing monomer. S2Cl2 provides improved miscibility with the co-monomers and is more reactive than elemental sulfur, which lowers the temperature required for polymerization.
The team polymerized S2Cl2 with different diallyl or triallyl-functionalized monomers by mixing the monomers and heating them to 45–120 °C. They obtained linear polymers, segmented block copolymers, and crosslinked thermoset materials. For example, the team successfully used the approach to prepare a new class of optically transparent polymers. According to the researchers, the method expands the scope of usable monomers compared with S8-based inverse vulcanization.
- Sulfenyl Chlorides: An Alternative Monomer Feedstock from Elemental Sulfur for Polymer Synthesis,
Kyung-Seok Kang, Chisom Olikagu, Taeheon Lee, Jianhua Bao, Jake Molineux, Lindsey N. Holmen, Kaitlyn P. Martin, Kyung-Jo Kim, Ki Hyun Kim, Joona Bang, Vlad K. Kumirov, Richard S. Glass, Robert A. Norwood, Jon T. Njardarson, Jeffrey Pyun,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c10317
Update (December 11, 2022)
The graphic was originally missing two carbon atoms; this has been corrected.